Answers
Related Questions
what mass of iron sulfide is obtained if 8 grams of sulfur and 28 grams of iron are taken for the reaction?
Answers
Mass of iron sulfide obtained : 22 g
Further explanationReaction
Fe+S⇒FeS
8 grams of sulfur and 28 grams of iron are taken for the reaction
Find limiting reactantmol of Fe(Ar=56 g/mol) :
\(\tt mol=\dfrac{mass}{Ar}\\\\mol=\dfrac{28}{56}\\\\mol=0.5\)
mol of S(Ar=32 g/mol) :
\(\tt mol=\dfrac{8}{32}=0.25\)
Limiting reactant ⇒ S(smaller mol ratio), excess reactant : Fe
mol of FeS based on limiting (S) ⇒0.25(mol ratio from equation = 1 : 1)
Mass FeS(MW=88 g/mol) :
\(\tt mass=mol\times MW\\\\mass=0.25\times 88\\\\mass=22~g\)
If a gas is cooled from 222 K to 125 K and the volume is kept constant, what would be the final
pressure if the original pressure was 760 mmHg?
Answers
We know the initial and final temperatures of the gas (T1 = 222 K; T2 = 125 K) and the initial pressure (760 mmHg). And we want to know what the final pressure would be. Since pressure and temperature are directly proportional and the final temperature is less than the initial temperature, we should expect the final pressure (P2) to be less than 760 mmHg.
Rearranging Gay-Lussac’s law to solve for P2, we obtain P2 = P1T2/T1. Plugging in our quantities, we get our final pressure: P2 = (760 mmHg)(125 K)/(222 K) = 428 mmHg.
How much time is required for an object moving 4.5m/s to travel 65 m
Answers
Answer:
About 14.45 seconds
Explanation:
If a object is going 4.5m/s and its destination is 65m then you need to divide.
65÷4.5 = 14.444444
due to the constant 4 you need to round 14.444 so it would be 14.45 seconds.
explain the relationship (linear or exponential) between rate and concentration including what order the iodate ion would be in.
CONCENTRATIONS
EXP. 1: 0.020
EXP 2: 0.019
EXP 3: 0.017
EXP 4: 0.016
EXP 5: 0.014
EXP 6: 0.013
EXP 7: 0.011
EXP 8: 0.01
EXP 9: 8.6x10^-3
EXP 10: 7.1x10^-3
EXP 11: 5.7x10^-3
EXP 12: 4.3x10^-3
RATE (s^-1):
EXP 1: 0.283
EXP 2: 0.1972
EXP 3: 0.2353
EXP 4: 0.2033
EXP 5: 0.1701
EXP 6: 0.133
EXP 7: 0.10
EXP 8: 0.1234
EXP 9: 0.077
EXP 10: 0.07380
EXP 11: 0.05102
EXP 12: 0.03883
By looking at the reaction mechanism, propose a Rate Law (WITHOUT the value of K). Explain the exponents for each reactant. Also, how does the rate law proposed compared to the relationship between rate and iodate concentration observed in the Rate law question?
Discuss, with respect to collision theory, the changes in the rates result from the changing concentrations of the iodate ion. What would you predict if we repeated these reactions at higher temperatures? Explain using collision theory.

Answers
Based on the given data, the relationship between rate and concentration is exponential.
A proposed rate law for the reaction based on the given data is:
Rate = k[IO3⁻]²[H+]What is the collision theory?Collision theory suggests that the rate of a chemical reaction is proportional to the frequency and energy of collisions between the reactant molecules.
As the concentration of iodate ions decreases, the frequency of collisions between reactant molecules decreases, which leads to a decrease in the rate of the reaction.
At higher temperatures, the kinetic energy of the reactant molecules increases, which increases the frequency and energy of collisions between reactant molecules.
Learn more about collision theory at: https://brainly.com/question/20628781
#SPJ1
Ammonium perchlorate is the solid rocket fuel used by the U.S. Space Shuttle. It reacts with itself to produce nitrogen gas , chlorine gas , oxygen gas , water , and a great deal of energy. What mass of water is produced by the reaction of of ammonium perchlorate? Be sure your answer has the correct number of significant digits.
Answers
Answer:
2.9 g of water are produced by 9.6 g of ammonium perchlorate
Note: The question is incomplete. The complete question is given below:
Ammonium perchlorate (NH₄ClO₄) is the solid rocket fuel used by the U.S. Space Shuttle. It reacts with itself to produce nitrogen gas (N₂) , chlorine gas (Cl₂), oxygen gas (O₂), water (H₂O) , and a great deal of energy. What mass of water is produced by the reaction of 9.6 g of ammonium perchlorate? Be sure your answer has the correct number of significant digits.
Explanation:
The reaction of ammonium perchlorate (NH₄ClO₄) to produce nitrogen gas (N₂) , chlorine gas (Cl₂), oxygen gas (O₂), water (H₂O) is shown in the balanced chemical equation given below:
2 NH₄ClO₄ → N₂ + Cl₂ + 2 O₂ + 4 H₂O
From the equation, 2 moles of ammonium perchlorate produces 4 moles of water, i.e. mole ratio of NH₄ClO₄ to H₂O = 2 : 4 = 1 : 2
molar mass of ammonium perchlorate, NH₄ClO₄ = (14 + 4 * 1 + 35.5 +16 * 4) = 117.5
molar mass of water, H₂O = (2 * 1 + 16) = 18.0 g
mass of water produced = moles of ammonium perchlorate * 2 * molar mass of water
moles of perchlorate = mass / molar mass = 9.6/117.5
mass of water produced = 9.6/117.5 * 2 * 18.0 g = 2.94 g of water
Therefore, 2.9 g of water are produced by 9.6 g of ammonium perchlorate
Suppose a student repeats Experiment 1 using strontium instead of magnesium. The student adds 4.93 g of strontium to a crucible, heats the crucible and its contents for several minutes over a Bunsen burner, and records the final mass of the crucible and its contents.
Write the balanced chemical equation for this reaction. Include physical states.
balanced equation:
What mass of product is expected to form in this reaction? Assume all of the strontium reacts.
mass of product:
Answers
The balanced chemical equation for the reaction between strontium and oxygen can be written as follows: 2 Sr (s) + \(O_2\)(g) → 2 SrO (s).
In this equation, solid strontium (Sr) reacts with gaseous oxygen (\(O_2\)) to produce solid strontium oxide (SrO).
To determine the mass of product expected to form in this reaction, we need to consider the molar ratio between strontium and strontium oxide. From the balanced equation, we can see that 2 moles of strontium react to produce 2 moles of strontium oxide.
The molar mass of strontium (Sr) is 87.62 g/mol, and the molar mass of strontium oxide (SrO) is 119.62 g/mol. Since the molar ratio is 1:1 between strontium and strontium oxide, the mass of strontium oxide formed will be equal to the mass of strontium used.
In this case, the student added 4.93 g of strontium to the crucible. Therefore, the expected mass of strontium oxide formed will also be 4.93 g.
It's important to note that this calculation assumes that the reaction proceeds to completion, meaning that all of the strontium reacts with oxygen. In actual laboratory conditions, the yield of the reaction may be less than 100% due to factors such as incomplete reaction, side reactions, or product loss.
For more such questsion on balanced chemical equation visit:
https://brainly.com/question/11904811
#SPJ8
List the 2 pKa's for H2SO4
Answers
The
is the wind system responsible for the movement of weather from west to east across most of the continental United States
A
polar easterlies
B
prevailing westerlies
C
trade winds
Answers
Answer:
Westerlies are prevailing winds that blow from the west at midlatitudes. They are fed by polar easterlies and winds from the high-pressure horse latitudes, which sandwich them on either side.Nov 15, C answer
Which of the following does not influence the effectiveness of a chemical sanitizer solution
Answers
The cost of a chemical sanitizer solution does not affect its effectiveness; option C.
What is a chemical sanitizer?A chemical sanitizer is a chemical which is designed to sanitize an environment or place by killing microorganisms which causes diseases or by rendering them inactive.
Some chemical sanitizers are used to sanitize laboratory work surfaces, hospitals floors and beds, as well as to sanitize the hands.
The factors that affect the effectiveness of a chemical sanitizer solution include:
ConcentrationTemperatureContact timeThe cost of a chemical sanitizer solution does not affect its effectiveness.
In conclusion, chemical sanitizers are used to sanitize surfaces.
Learn more about chemical sanitizers at: https://brainly.com/question/27147558
#SPJ1
Note that the complete question is as follows:
Which of the following does NOT influence the effectiveness of a chemical sanitizer solution?
Concentration
Temperature
Cost
Contact time
Compute the number of electrons that each lead atom donates, on average, to a bulk piece of lead metal. Room temperature data for lead: The conductivity of lead is 4.90 × 104 1/(Ω·m) The electron mobility of lead is 2.3 cm2/(V·s) The mass density of lead is 11.4 g/cm3 The atomic weight of lead is 207 g/mol
Answers
Answer:
4 electrons/atom
Explanation:
The conductivity of the lead σ = neμ where n = electron density, e = electron charge = 1.602 × 10⁻¹⁹ C and μ = electron mobility of lead = 2.3 cm²/(V·s) = 2.3 × 10⁻⁴ m²/(V.s)
Making n subject of the formula, we have
n = σ/eμ
Since σ = 4.90 × 10⁶ (Ω·m)⁻¹
Substituting the values of the variables into the equation, we have
n = σ/eμ
n = 4.90 × 10⁶ (Ω·m)⁻¹/(1.602 × 10⁻¹⁹ C × 2.3 × 10⁻⁴ m²/(V.s))
n = 4.90 × 10⁶ (Ω·m)⁻¹/(3.6846 × 10⁻²³ Cm²/(V.s))
n = 1.33 × 10²⁹ electrons/m³
We now find the number of moles of lead present in 1 m³ of lead.
So n' = ρ/M where ρ = density of lead = 11.4 g/cm³ = 11.4 g/cm³ × 10⁶ cm³/m³ = 11.4 × 10⁶ g/m³ and M = atomic weight of lead = 207 g/mol
So, n' = ρ/M
N = 11.4 × 10⁶ g/m³/207 g/mol
n' = 0.0551 × 10⁶ mol/m³
n' = 5.51 × 10⁴ mol/m³
Since n' = N/N' where N = number of atoms of lead in 1 m³ of lead and N = Avogadro's constant = 6.022 × 10²³ mol⁻¹
N = n'N' = 5.51 × 10⁴ mol/m³ × 6.022 × 10²³ mol⁻¹
N = 33.18 × 10²⁷ atoms/m³
N = 3.318 × 10²⁸ atoms/m³
So, the number of electron per atom is N" = n/N
= 1.33 × 10²⁹ electrons/m³ ÷ 3.318 × 10²⁸ atoms/m³
= 0.4 × 10¹ electrons/atom
= 4 electrons/atom
6. How is the pressure of a gas affected by temperature changes? Assume no change in volume or mass in your explanation.
Answers
Of the following regions of the electromagnetic spectrum, which one has the shortest wavelength?
a.
gamma rays
b.
infrared
c.
radio waves
d.
X rays
e.
microwaves
f.
ultraviolet
Answers
Answer:
A ---->gamma ray
Explanation:
Gamma rays have the highest frequencies among all electromagnetic waves and therefore have the shortest wavelengths.
How many atoms of hydrogen are in 2.89 x 10^21 molecules of tryptophan (C11H12N2O2)?
Answers
1) Atoms to molecule ratio
1 molecule of C11H12N2O2: 12 atoms of H
2) Converting molecules of C11H12N2O2 to atoms of H.
\(\text{atoms of H=2.89}\cdot10^{21}C_{11}H_{12}N_2O_2\cdot\frac{12\text{ atoms of H}}{1moleculeofC_{11}H_{12}N_2O_2}=3.468\cdot10^{21}atomsofH_{}\)There are 3.468*10^21 atoms of H.
The strength of mild steel is found to be 232.9 MPa when the grain size is 17.43 pm. and 874.2 MPa when the grain size is 0.80 um. 1. Determine the constants in the Hall-Petch equation. (Express your answer to three significant figures.) K =_____MPa um 0o =______MPa 2. Determine the strength of the mild steel when the grain size is reduced to 0.160 pm. (Express your answer to three significant figures.) 0 = MPa
Answers
When the grain size is reduced to 0.160 pm, the strength of mild steel is 1851 MPa.There are a number of variables that can affect mild steel's strength, including the grain size, which is controllable.
We may use the above information to determine the constants in the Hall-Petch equation: The strength of mild steel at grain size d1 = 17.43 pm is 1 = 232.9 MPa. Mild steel has a strength of 2 = 874.2 MPa when the grain size is d2 = 0.80 um (or 800 nm). This is the formula for the Hall-Petch equation: = o + Kd(-1/2). We can use the following equation to determine K: K = (σ2 - σ1)(d2^(1/2) - d1^(1/2))^(-1) (-1) When the values are plugged in, we get the following result: K = (874.2 - 232.9)(800(1/2) - 17.43(1/2))(-1) = 276.3 MPa um(1/2) We can use the following equation to determine o: σo = σ1 - Kd1^(-1/2) Plugging in the numbers yields the following result: o = 232.9 - 276.3(17.43(-1/2)) = 62.28 MPa. The Hall-Petch equation's constants are K = 276.3 MPa um(1/2) and o = 62.28 MPa. Hence, the power of the yield strength of mild steel at 0.160 pm grain size is 1851 MPa.
Learn more about mild steel here:
https://brainly.com/question/30036139
#SPJ4
Insert the term that correctly completes the paragraph.
Illustration of a rock showing that the layers are not stacked horizontally and look like a rainbow of layers.
Soledad studied rocks and how they help show the history of Earth. She knew that scientists use different ways to find out how old rock layers are and recognized one such example in the rock in the image. The image is an example of a/an
rock.
Answers
Gravity causes spacecraft such as stars, planets, moons, and other bodies to orbit one another. Revolution describes this style of movement.
What is Gravity ?Gravity causes spacecraft such as stars, planets, moons, and other bodies to orbit one another. Revolution describes this style of movement. Space is also home to several stationary things. Rotation describes this movement.We experience day and night because of how long it takes the Earth to complete one rotation. A year is the length of one Earth rotation around the sun. Due to their varying rates of rotation and revolving, other planets have days and years that differ from our own.In the same direction, every planet in our solar system orbits the sun. In addition, the majority of them rotate in the same direction (with the exception of Venus and Uranus). In the cosmos, approximately half of all galaxies rotate in a clockwise direction, and the other half in a counterclockwise direction. This may be due to the manner that the cosmos started, according to scientists.To Learn more About Gravity refer To:
https://brainly.com/question/557206
#SPJ1
Compared to an enzyme with a Km of 10-6 mM, an enzyme having a Km of 10-8 mM
A. would have 100 times more product formed per min
B. would be described as having binding more tightly to the substrate
C. would be described as having a significantly lower affinity for the substrate
D. would have a Vmax 100 times larger
E. would have identical values for Vmax
Answers
Answer:
The correct answer is option B - would be described as having binding more tightly to the substrateExplanation:
Compared to an enzyme with a Km of 10-6 mM, an enzyme having a Km of 10-8 mM
The correct answer is option B - would be described as having binding more tightly to the substrate
Reason:
Km is the measure of substrate affinity. It is inversely proportional. Higher the Km, lower is the binding affinity of the enzyme and lower the Km, higher binding affinity of the enzyme to the substrate.
Therfore, Km of 10⁻⁸ will have higher substrate binding affinity than the 10⁻⁶ enzyme.
How much heat is required to melt 20 grams of ice? (delta Hfus of ice = 6.01kJ/mol)
Answers
6.67 kJ of heat is required to melt 20 grams of ice. To calculate the amount of heat required to melt a certain amount of ice.
we need to use the formula:
Q = n * ΔH_fus
where Q is the amount of heat required, n is the number of moles of ice, and ΔH_fus is the heat of fusion of ice.
To find the number of moles of ice in 20 grams, we need to divide the mass by the molar mass of ice. The molar mass of water is 18.015 g/mol.
n = m / M = 20 g / 18.015 g/mol = 1.110 mol
Now we can use the formula above to calculate the amount of heat required:
Q = n * ΔH_fus = 1.110 mol * 6.01 kJ/mol = 6.67 kJ
Therefore, 6.67 kJ of heat is required to melt 20 grams of ice.
To learn more about grams please click on below link.
https://brainly.com/question/11260752
#SPJ1
A large balloon contains 5400 m3 of He gas that is kept at a temperature of 280 K and an absolute pressure of 1.10 x 105 Pa. Find the mass of He inside the balloon. (Molar mass of He : 4.002 g/mol)
Answers
Answer:
1.02 × 10⁶ g
Explanation:
Step 1: Given data
Volume of the balloon (V): 5400 m³Temperature (T): 280 KAbsolute pressure (P): 1.10 × 10⁵ PaMolar mass of He (M): 4.002 g/molStep 2: Convert "V" to L
We will use the conversion factor 1 m³ = 1000 L.
5400 m³ × 1000 L/1 m³ = 5.400 × 10⁶ L
Step 3: Convert "P" to atm
We will use the conversion factor 1 atm = 101325 Pa.
1.10 × 10⁵ Pa × 1 atm / 101325 Pa = 1.09 atm
Step 4: Calculate the moles of He (n)
We will use the ideal gas equation.
P × V = n × R × T
n = P × V / R × T
n = 1.09 atm × 5.400 × 10⁶ L / 0.08206 atm.L/mol.K × 280 K
n = 2.56 × 10⁵ mol
Step 5: Calculate the mass of He (m)
We will use the following expression.
m = n × M
m = 2.56 × 10⁵ mol × 4.002 g/mol
m = 1.02 × 10⁶ g
The liquid that condenses during distillation is called?
Answers
Answer:
the answer is distillate
Net ionic equation for potassium sulfide and magnesium iodide
Answers
The net ionic equation for the reaction between potassium sulfide and magnesium iodide is S2- + Mg2+ -> MgS, as the potassium and iodide ions are spectator ions and do not participate in the reaction.
To determine the net ionic equation for the reaction between potassium sulfide (K2S) and magnesium iodide (MgI2), we first need to identify the ions present in each compound and then determine the products formed when they react.
Potassium sulfide (K2S) dissociates into two potassium ions (K+) and one sulfide ion (S2-):
K2S -> 2K+ + S2-
Magnesium iodide (MgI2) dissociates into one magnesium ion (Mg2+) and two iodide ions (I-):
MgI2 -> Mg2+ + 2I-
Now, we need to determine the possible products when these ions combine. Since potassium (K+) has a +1 charge and iodide (I-) has a -1 charge, they can combine to form potassium iodide (KI):
K+ + I- -> KI
Similarly, magnesium (Mg2+) and sulfide (S2-) can combine to form magnesium sulfide (MgS):
Mg2+ + S2- -> MgS
Now, we can write the complete ionic equation by representing all the ions present before and after the reaction:
2K+ + S2- + Mg2+ + 2I- -> 2KI + MgS
To obtain the net ionic equation, we remove the spectator ions, which are the ions that appear on both sides of the equation and do not participate in the actual reaction. In this case, the spectator ions are the potassium ions (K+) and the iodide ions (I-).
Thus, the net ionic equation for the reaction between potassium sulfide and magnesium iodide is:
S2- + Mg2+ -> MgS
For more such questions on ionic equation visit:
https://brainly.com/question/25604204
#SPJ8
A balloon contains 0.76 mol O2, 0.18 mol Ne, 0.031 mol Xe and 0.026 mol CO2 at 749 mm Hg. What is the partial pressure of Ne
Answers
Answer:
ans given below
Explanation:
To keep simplicity, let's assume these gases behave ideally so we can use the equations for ideal gas. One of these equation is the Raoult's Law written below.
P* = xP
This is another form of the Raoult's Law where x is the mole fraction. Let's find x for O2.
x = 0.18/(0.76+0.18+0.031+0.026) = 0.1805
Partial Pressure = (0.1805)(749 mmHg) = 135.23 mmHg
The pOH of a solution is 6.0. Which statement is correct?
Use pOH = -log[OH-] and PH+pOH = 14.
The pH of the solution is 20.0.
O The concentration of OH ions is 1.0 x 108 M.
The concentration of OH ions is 1.0 x 106 M.
O The pH of the solution is 8.0.
A
Answers
At pOH value of 6.0 the pH value of the following solution is 8.0 and the concentration of [\(H^{+}\) ] ion is \(10^{-8}\)
In this question we will apply the formula
pH +pOH = 14 . . . . . . . . . . . . .(1)
where pH = concentration of [\(H^{+}\) ] ion
pOH = concentration of [\(OH^{-}\) ] ion
As per the question
pOH =6.0
Putting the value of pOH in equation (1) we get the value of pH
pH + 6.0 =14
pH = 14 -6.0
pH = 8.0
The value of pH if the pOH value is 6.0 is 8.0
To find the concentration of \(H^{+}\) ion we will use the following formula
This is calculated by the formula
[\(H^{+}\)} = \(10^{-pH}\)
where we will write the values of pH
Hence the concentration of [\(H^{+}\)} ion is \(10^{-8}\)
Therefore at pOH of 6.0 the pH value of the following solution is 8.0 and the concentration of [\(H^{+}\) ] ion is \(10^{-8}\)
Read more about pH
https://brainly.com/question/11300720
The complete question is -
What is the pH value and concentration of [\(H^{+}\) ] ion of the following if the pOH value of the solution is 6.0 ?
Without checking the detailed numbers, please arrange the ionic compounds CsI, MgO, NaCl, and AlN in order of increasing lattice energy.
Answers
For the above compounds, the order of increasing lattice energy is MgO, NaCl, CsI, and AlN.
Which is ionic? MgO or NaCl?Because the ionic species in MgO have greater charge (Mg+2 and O2- as opposed to Na+ and Cl-), the ionic bond is stronger than it is in NaCl. As a result, MgO has greater ionic connections than NaCl. MgO is hence more ionic than NaCl.
whose MgO lattice energy is the highest?The size of the ions involved has an inverse relationship with lattice energy in ionic compounds. MgO has the highest lattice energy because Mg2+, the smallest of the four ions (because anion is the same in all), is present.
To know more about lattice energy visit:-
https://brainly.com/question/18222315
#SPJ1
How much would 400. jelly beans weigh in grams?
Answers
Answer:
453 or 453.592
Explanation:
400 jellybeans are about a pound and a pound is 453.592 grams
Why does a gas smell diffuse faster than a colour in a liquid?
Answers
Answer:
because there are other particles to help it move around in the air
Calculate the standard entropy change
C2H2 (g) + 2H2 (g) → C2H6 (g
C2H2= 201
H2=131
C2H6 = 230
Answers
Entropy is a notion that essentially refers to the universe's propensity for chaos or the spontaneous changes that take place in everyday happenings. Here the standard entropy change for the given reaction is -233.
Entropy is typically referred to as a measurement of a system's randomness or disorder. In the year 1850, a German physicist by the name of Rudolf Clausius first proposed this idea. Entropy is a thermodynamic property that is used to characterize how a system behaves in terms of temperature, pressure, entropy, and heat capacity.
Here the standard entropy change is:
Entropy of products - entropy of reactants
ΔS = 230 - (201 + 2 ( 131)) = -233
To know more about entropy, visit;
https://brainly.com/question/17172535
#SPJ1
What is the mass number of an element?
A. Mass number is the number of protons in the nucleus.
B. Mass number is the mass of the protons in the nucleus.
C. Mass number is the mass of the most abundant isotope.
D. Mass number is the atomic mass of a particular isotope.
Answers
The mass number of an element is the sum of the protons and neutrons present in the nucleus of the atoms of the element.
What is mass number?The mass number of an element is the number obtained when the number of protons and neutrons in the nucleus of an atom of the element are summed together.
The sum of protons and neutrons in the nucleus of an atom is collectively known as the nucleon. Thus, the mass number of an atom can also be referred to as the nucleon of the atom.
This can be mathematically expressed as:
Mass number = number of protons + number of neutrons.
Atoms generally contain protons, neutrons, and electrons. The protons are positively charged and are located in the nucleus, the neutrons are also located in the neucleus but have no charges. The electrons, on the other hand, are located outside the nucleus in regions referred to as orbitals.
The sum of the protons and neutrons determine the mass of an atom because the contribution of electrons to the mass of atoms is negligible.
Thus, the mass number of an element is the sum of proton and neutron numbers present in the nucleus of the atoms of the element.
More on mass number can be found here: https://brainly.com/question/4408975
#SPJ1
May someone help me please ASAP !
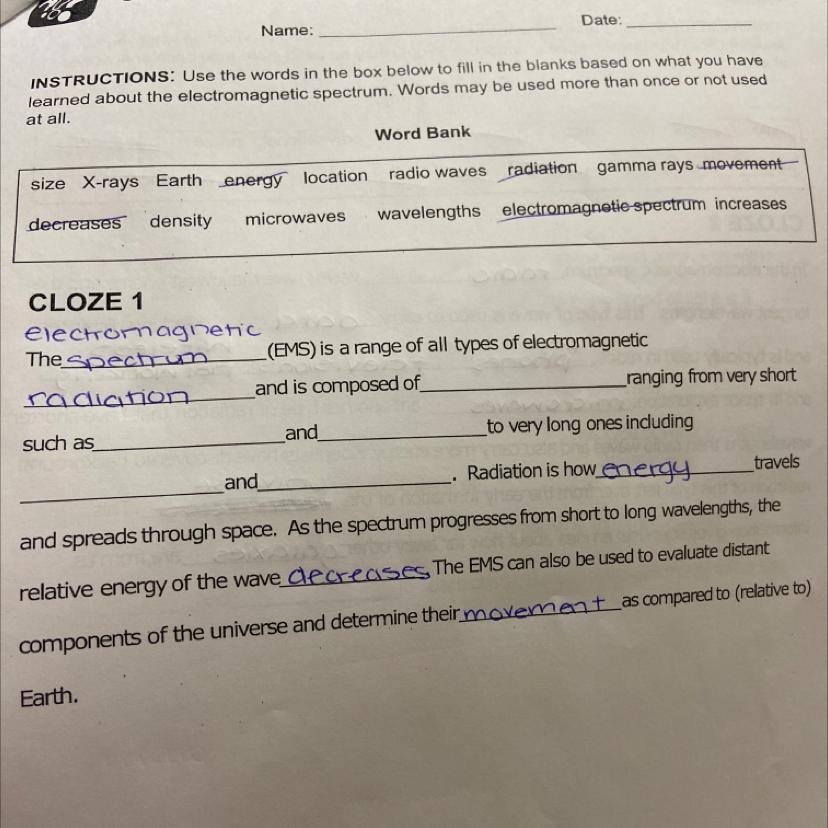
Answers
Answer:
is composed of radio waves
such as x-rays and gamma rays
microwave and radio waves
if am not mistakes this might help
2.
Which mixture could be a useful buffer in a solution?
acetic acid (CH3CO2H) and hydrochloric acid (HCl)
sodium hydroxide (NaOH) and elemental sodium (Na)
ammonia (NH3) and ammonium chloride (NH4Cl)
acetic acid (CH3CO2H) and ammonia (NH3)
Pls answer quickly
Answers
Ammonia (\(NH_3\)) and ammonium chloride (\(NH_4Cl\)) mixture could be a useful buffer in a solution. Option C
A buffer is a solution that can resist changes in pH when small amounts of acid or base are added. It consists of a weak acid and its conjugate base or a weak base and its conjugate acid. The buffer system works by the principle of Le Chatelier's principle, where the equilibrium is shifted to counteract the changes caused by the addition of an acid or a base.
In option A, acetic acid (\(CH_3CO_2H\)) is a weak acid, but hydrochloric acid (HCl) is a strong acid. This combination does not form a buffer because HCl is completely dissociated in water and cannot provide a significant concentration of its conjugate base.
Option B consists of sodium hydroxide (NaOH), which is a strong base, and elemental sodium (Na), which is a metal. This combination does not form a buffer as there is no weak acid-base pair involved.
Option D contains acetic acid (\(CH_3CO_2H\)), a weak acid, and ammonia (\(NH_3\)), a weak base. Although they are weak acid and base, they do not form a buffer system together as they are both weak acids or bases and lack the required conjugate acid-base pair.
Option C, ammonia (\(NH_3\)), is a weak base, and ammonium chloride (\(NH_4Cl\)) is its conjugate acid. This combination can form a buffer system. When ammonia reacts with water, it forms ammonium ions (NH4+) and hydroxide ions (OH-).
The ammonium ions act as the weak acid, while the ammonia acts as the weak base. The addition of a small amount of acid will be counteracted by the ammonium ions, and the addition of a small amount of base will be counteracted by the ammonia, thus maintaining the pH of the solution relatively stable.
Therefore, option C, consisting of ammonia (\(NH_3\)) and ammonium chloride (\(NH_4Cl\)), is the suitable mixture that could be a useful buffer in a solution.
For more such question on buffer visit:
https://brainly.com/question/13076037
#SPJ8
Which of these pairings with create an octet for each atom?
A. One aluminum atom and one oxygen atom
B. One magnesium and one chlorine
C. One magnesium and one oxygen
D. One potassium and one sulfur atom
Answers
Answer:
C) one magnesium and one oxygen