What two weak interactions are most important in the formation of a cluster of lipid molecules in an aqueous solution?
Answers
The two weak interactions that are most important in the formation of a cluster of lipid molecules in an aqueous solution are hydrophobic interactions and van der Waals interactions.
Hydrophobic Interactions: Lipids are amphiphilic molecules, meaning they have both hydrophilic (water-loving) and hydrophobic (water-fearing) regions. In an aqueous solution, such as water, the hydrophobic regions of lipid molecules tend to cluster together to minimize their exposure to water.
This clustering is driven by hydrophobic interactions, which are weak forces that arise due to the disruption of the hydrogen bonding network of water molecules around the hydrophobic lipid tails. By clustering together, the lipid molecules can reduce the contact between their hydrophobic regions and water, thereby minimizing the unfavorable disruption of the water structure.
Van der Waals Interactions: Van der Waals interactions refer to the weak attractive forces that occur between all molecules, including lipids. These interactions arise due to temporary fluctuations in electron distribution, creating temporary dipoles in one molecule that can induce dipoles in neighboring molecules.
In the case of lipid molecules, van der Waals interactions play a significant role in stabilizing the lipid clusters. The hydrophobic tails of adjacent lipid molecules can experience attractive van der Waals forces, promoting the close packing of lipids within the cluster.
These two weak interactions, hydrophobic interactions and van der Waals interactions, are crucial for the formation and stability of lipid clusters in an aqueous solution. They contribute to the self-assembly of lipids into various structures, such as micelles, lipid bilayers, or liposomes, which are essential for biological processes and the formation of cell membranes.
know more about hydrophobic here
https://brainly.com/question/31824394#
#SPJ11
Related Questions
Earth’s layers are determined by their physical properties and chemical composition. In the lesson, you learned about each of Earth’s layers, what the layers are made of, and why they are important to the Earth. In the space below, write about how the atmosphere may be classified. Would it be part of the lithosphere, or a separate layer? Explain your answer.
Answers
Answer:
The Atmosphere Is a separate layer And Also could be classified as the Closest layer
Explanation:
_______ oxides are oxides of certain non metals
Answers
acidic in naturenature
Generally, Oxides of non-metals are acidic in nature. When non-metals are reacted with water it forms an acidic solution. The common oxides of Sulphur, Selenium, and Bromine are strongly acidic.
Consider the following balanced reaction between hydrogen and nitrogen to form ammonia:
3H2(g) + N2(g) → 2NH3(g)
How many moles of NH3 can be produced from 29.0 mol of H₂ and excess N₂?
Express the number of moles to three significant figures.
Answers
The number of moles of NH3 that can be produced is 19.3 moles.
Stoichiometric problemFrom the balanced equation of the reaction, the mole ratio of H2 reacted and NH3 produced is 3:2.
This means that for every 1 mole of H2 that reacts, 2/3 mole of NH3 will be produced.
Now, we have 29.0 moles of H2 reacting. If 1 mole of H2 produces 2/3 moles NH3, then 29 moles of H2 will produce:
29 x 2/3 = 19.3333 moles of NH3
To three significant figures, 19.333 moles will be 19.3 moles.
More on stoichiometric problems can be found here: https://brainly.com/question/14465605
#SPJ1
What are the criteria of high-quality scientific research?
Provide at least three examples and explain them in detail.
Answers
High-quality scientific research is characterized by several key criteria. Three examples of such criteria include: rigorous experimental design and methodology, reliable data analysis and interpretation, and clear and transparent reporting of results.
These criteria ensure that research is conducted in a systematic and reliable manner, leading to trustworthy and valid findings.
Rigorous Experimental Design and Methodology: High-quality scientific research requires a well-designed experimental approach. This involves careful planning, proper control groups, randomization, and replication. A rigorous methodology ensures that experiments are conducted under controlled conditions, minimizing bias and confounding variables, and allowing for accurate and reliable data collection.
Reliable Data Analysis and Interpretation: After data collection, high-quality research involves thorough and appropriate analysis of the data. This includes using appropriate statistical methods to evaluate the significance of the results and drawing valid conclusions. Proper data analysis helps researchers identify patterns, trends, and relationships, supporting or refuting their hypotheses in an objective and reliable manner.
Clear and Transparent Reporting of Results: High-quality research demands transparent reporting of the methods, procedures, and findings. This includes providing detailed descriptions of the experimental setup, data collection processes, and statistical analyses used. Clear reporting allows other researchers to replicate the study and verify its results. Additionally, complete reporting ensures that readers can understand the research methodology and draw their own conclusions based on the evidence presented.
By adhering to these criteria, high-quality scientific research maintains integrity, credibility, and reproducibility. It fosters trust among the scientific community and facilitates the advancement of knowledge by building upon reliable foundations.
To learn more about research click here:
brainly.com/question/31251355
#SPJ11
Liquid to Gas =
Solid to Liquid =
Answers
The process of a liquid becoming a gas is called boiling (or vapourization) and the process of a solid becoming a liquid is called melting.
Vaporization is the process of converting a liquid into a gas. It is also called evaporation. Since we know that the particles of a gas are moving faster than those of a liquid, an input of energy must be required for a liquid to become a gas.
Melting is a physical process that results in the phase transition of a substance from a solid to a liquid. During melting, the energy goes exclusively to changing the phase of a substance; it does not go into changing the temperature of a substance. Hence melting is an isothermal process because a substance stays at the same temperature.
Example 1: Industrially, salt is recovered from seawater by the process of vaporization. Wet clothes are dried up due to the process of vaporization. The process is used in many industrial processes for separating the components of a mixture.
Example 2: Ice to water - Ice melts back into the water when it is left out at temperatures above the freezing point of 32 degrees. Rocks to lava - Rocks in volcanoes can be heated until they are molten lava. Metal to molten liquid - Metals such as steel and bronze can be molten down.
To learn more about vaporization and melting visit,
https://brainly.com/question/17945501
a student performing the qual group 1 and 2 unknown analysis did not have any precipitate remaining after step 1-a. what steps should the student omit from the procedure? explain your answer.
Answers
In qualitative analysis, the student should omit the steps 1B-1E which leads us to omitting steps which involves the presence of Pb and Ag.
What is qualitative analysis?
In qualitative analysis, information that is non-numerical about a chemical species, a reaction, etc. are determined. Qualitative analysis is not a reliable analysis but it often easier, faster and is a lot cheaper to perform that the quantitative analysis. Qualitative Analysis deals with the grouping or identification of elements present in the sample. It is a standard procedure to classify the methods in two classes and those are qualitative inorganic analysis and qualitative organic analysis.
Hence, In qualitative analysis if there is no precipitate, student should omit the step 1B-1E.
To know more about qualitative analysis, follow the below link
https://brainly.com/question/15858232
#SPJ4
The student should omit steps 1B through 1E, which leads us to omit steps involving the presence of Pb and Ag, when performing a qualitative analysis.
What is qualitative analysis?
Qualitative analysis is used to determine non-numerical information about a chemical species, a reaction, etc. Although qualitative analysis is less accurate than quantitative analysis, it is frequently quicker, simpler, and much less expensive to conduct. The grouping as well as identification of elements found in the sample is the focus of qualitative analysis. The methods are typically divided into two categories: qualitative inorganic analysis as well as qualitative organic analysis.
Hence, In qualitative analysis if there is no precipitate, student should omit the step 1B-1E.
To know more about qualitative analysis, follow the below link
brainly.com/question/15858232
#SPJ4
Anybody good at chemistry that is willing to help?? If you could that'd be greatly appreciated!
(25 PTS)

Answers
Answer:
The least similar to a real gas found in the environment would be B.
Explanation:
Standard Ambient Temperature/Pressure (SATP) is at 298.15K and 101.3kPa. It describes standard conditions and is often used for measuring gas density and volume using the Ideal Gas Law. The values least similar to theses ones would be the least similar in the gases in the environment.
Need organic chemistry 11th grade
for practice and 1 question is enough :)
Answers
Answer:
ok all you need is a little study
Explanation:
organic chemistry isn't something to worry about is something that is a very simple concept if you understand what you're doing so a little bit of studying will help you go through it and you really don't this is not the topics that you will really understand if you just go through it as once so there is no need to worry about organic chemistry it is very simple concept just learning you are a you are close just learn all those simple aspects alkane etc it's very easy
How do slab pull forces affect plate motion? Choose the correct answer.
Uplifted mantle causes plates to move apart.
Convection currents drive the plate downward to move it.
Earthquakes break the crust into smaller plates to make motion easier.
The weight of the subducted plate produces a force to continue moving the plate.
Answers
Answer:
The weight of the subducted plate produces a force to continue moving the plate.
Explanation:
2H2O + 3HCl ——> 5H2O + Cl
how many moles of H2O are needed to make 3 moles of HCl?
Answers
Answer: 2 moles
Explanation:
We know from the equation that for every 3 moles of HCl consumed, 2 moles of water are consumed (based on the coefficients)
So, the answer is 2 moles.
Una solución hallar punto de vista física y punto de vista químico (sal) 50g de sal y 250 ml de agua hallar %m/m %m/v molalidad, molaridad, fracción molar del soluto y del solvente
Answers
Answer:
% m/m = 16.6%
% m/v = 20%
3.42 m (molalidad)
3.42 M (molaridad)
Fraccion molar del soluto = 0.06
Fraccion molar del solvente = 0.94
Explanation:
Nuestra solución está compuesta por:
Soluto: 50 g de sal
Solvente: 250 mL de agua.
%m/m %m/v molalidad, molaridad, fracción molar del soluto y del solvente son distintas formas de definir la concentración:
% m/m son los gramos de soluto cada 100 g de solución.
No sabemos la masa de solución pero con la densidad del agua (solvente), sabemos que los 250 mL contienen 250 gramos de solvente. (Densidad del agua 1g/mL). En conclusión, la masa de solución es 50 g + 250g = 300
% m/m = (50g /300g ) . 100 = 16.6%
Vamos a definir que el volumen de la solución es igual al del solvente: 250 mL.
% m/v = la masa de soluto, cada 100 mL de solución
= (50 /250) . 100 = 20 %
Molalidad, son los moles de soluto por cada kg de solvente.
Convertimos gramos a kg → 250 g . 1kg /1000g = 0.25kg
Convertimos la masa de soluto (sal) a moles.
NaCl → 50 g . 1mol/ 58.45g = 0.855 moles
m = 0.855 /0.25 → 3.42 m (molalidad)
Molaridad son los moles de soluto por cada L de solución.
Convertimos mL a L → 250 mL . 1kg / 1000mL = 0.25 L
sabiendo que los moles de soluto son 0.855, en este caso la molaridad es igual a la molalidad ( 3.42 M)
Para calcular la fracción molar, necesitamos moles de soluto y de solvente.
Ya contamos con los moles de soluto.
Moles de solvente: 250 g . 1mol /18g = 13.89 mol
Moles totales = 0.855 + 13.89 = 14.745
Fracion molar del soluto: (0.855/ 14.745) = 0.06
Fraccion molar del solvente: (13.89 / 14.745) = 0.94
if all the n2 and h2 are consumed, what volume of nh3 , at the same temperature and pressure, will be produced?
Answers
0 degrees Celsius or one atmosphere are considered the norms for both temperature and pressure.A molar gas volume in STP is 22.4 liters / mole, according to what is reported.
What is relation between temperature and pressure?We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then P and T are directly proportional (again, when volume and moles of gas are held constant).
What happens to pressure when temperature increases?As the temperature increases, the average kinetic energy increases as does the velocity of the gas particles hitting the walls of the container. The force exerted by the particles per unit of area on the container is the pressure, so as the temperature increases the pressure must also increase.
To know more about temperature and pressure visit:
https://brainly.com/question/12128633
#SPJ4
Thorium 234 has a half-life of 24.1 days. Will all the thorium atoms in a sample decay in 48.2 days? Explain
Answers
Answer:
No, it will still have 58.8 g of mass
Explanation:
If the half-life of Thorium 234 is24.1 days, then 24.1 days have passed, Thorium will lose half its mass. Its initial mass is 234, so after 24.1 days it will be halved: 117
If pass more 24.1 days, it willlost the half of its mass again, so the half of 117: 58.5.
If you add the days, 24.1 + 24.1, it will give 48.2. So the sample will still have 58.5 of mass before 48.2 days.
Thorium 234 is a radioactive element which keeps on decaying over the time. Decay of radioactive element always comes under first order kinetics. Therefore after 48.2 days Thorium 234 will not be completely decayed.
What is half life?Half life tells about the time at which the radioactive material decays to half of its initial concentration.
Mathematically,
\(t_{1/2} = \frac{0.693}{\lambda}\)
\(t_{1/2}\) is the half life time
\(}{\lambda}\) is the rate constant of the decay
If the half-life of Thorium 234 is24.1 days, then Thorium will lose half of its mass. Its initial mass is 234, so after 24.1 days it will be halved that is 117. If we add another half life time, then again it will loose half of its mass, so the half of 117 is 58.5. On adding 24.1 + 24.1, we get 48.2. So the sample will still have 58.5 of mass after 48.2 days.
Therefore after 48.2 days Thorium 234 will not be completely decayed.
To know more about half life, here:
https://brainly.com/question/21464063
#SPJ2
please help with these 5 questions if they physical or chemical as well as intensive or extensive i will be giving brainliest thank u

Answers
Before categorizing each of the properties into physical or chemical, intensive or extensive, we need to understand the terms:
Physical property: This is any characteristic that can be determined without changing the substance’s chemical identity.
Chemical property: This is any characteristic that can be determined by simply changing the molecular structure of a substance
1) For the property: Its melting point is 112 degrees Celcius is a Physical property and intensive since extensive property depends on the amount of matter being measured.
2) If a substance burns in a presence of oxygen, it is a chemical property since the molecular structure of the resulting compound will be changed. This is also an intensive property.
3) If a substance is yellow solid is a physical property since the first is the color yellow and solid shows the state of the element. Neither property implies that the substance is changing its composition. Therefore, both properties are physical properties. This physical property is Intensive properties since it does not depend on the amount of the substance present.
4) If the substance is combustible, it is a chemical property and intensive property
5) For the sample of a substance that measures 75cm^3, this is a Physical property since the volume of a substance can be determined without changing its identity. This is also an extensive property since examples of these properties include mass, weight, and volume.
One major problem with wind and solar energy is that they are conditional. Explain how hydroelectric pumped storage could be used to eliminate this
obstacle? *
Link to the article https://thinkprogress.org/the-inside story-of-the-worlds-biggest-battery-and-the-future-of-renewable-energy-8984c81283c/
Answers
Answer:
Hydroelectric pumped storage can be used to eliminate the conditional nature of wind and solar energy. At times of peak production of energy from either solar or wind (during summer and windy days), the excess electrical energy produced can be stored using hydroelectric pumped storage methods. When conditions no longer favour energy production from either wind or solar sources, these stored energy can then be regenerated for use.
Explanation:
Pumped-storage hydroelectricity is a type of hydroelectric energy storage used by electric power systems to store excess electrical power during periods of low demand for later release at periods when demand for energy rises again. It stores energy in the form of gravitational potential energy of water, pumped from a lower level reservoir to a higher level reservoir.
At times of low electrical demand, excess generated energy is used to pump water into the upper reservoir. When there is higher demand, water is released back into the lower reservoir through a turbine, generating electricity.
This form of energy storage is useful in circumventing the conditional nature of renewable energy sources such as wind and solar energy. At times of peak production of energy from either solar or wind (during summer and windy days), the excess electrical energy produced can be stored using hydroelectric pumped storage methods. When conditions no longer favour energy production from either wind or solar sources, these stored energy can then be regenerated for use.
carbon dioxide and particulates are emitted by volcanoes. particulates form stratospheric aerosols that reflect sunlight. which of the following best describes the impact of atmospheric carbon dioxide and stratospheric aerosols?
Answers
Carbon dioxide and particulates are emitted by volcanoes. Particulates form stratospheric aerosols that reflect sunlight The impact of atmospheric carbon dioxide and stratospheric aerosols can be briefly described below.
Carbon dioxide (CO₂): Carbon dioxide is a greenhouse gas that contributes to the greenhouse effect and global warming. It is released into the atmosphere through various human activities, such as the burning of fossil fuels and deforestation. The increasing concentration of CO₂ in the atmosphere is a significant driver of climate change.
Stratospheric aerosols: Stratospheric aerosols, formed by the release of particulates from volcanic eruptions or human activities, can have a cooling effect on the Earth's climate. These aerosols reflect sunlight back into space, reducing the amount of solar radiation that reaches the Earth's surface. As a result, they can temporarily offset some of the warming caused by greenhouse gases like CO2.
It's important to note that while stratospheric aerosols can have a cooling effect, they are relatively short-lived in the atmosphere compared to CO₂. Carbon dioxide, on the other hand, has a long residence time and accumulates over time, leading to long-term warming.
Therefore, the overall impact of increasing atmospheric CO₂ concentrations outweighs the cooling effect of stratospheric aerosols.
Learn more about stratospheric aerosols here:
https://brainly.com/question/28073358
#SPj 4
WHAT IS THE UNBALCNED EQUATION OF Methane burns in oxygen to produce carbon dioxide and water
Answers
Answer:
\(\boxed{CH_{4}+O_2 --> CO_2 + H_2O}\)
Explanation:
Part 1: Naming compound formulas given the namesStep 1. Methane's formula is \(CH_4\).
Step 2: Oxygen is a diatomic molecule (it exists bonded to itself for stability purposes), so by itself in chemical equations, it is written as \(O_2\).
Step 3: Carbon dioxide is the molecular compound of one atom of carbon and two atoms of oxygen → \(CO_2\).
Step 4: Water is the common name of the compound of two hydrogen atoms and one oxygen atom → \(H_2O\).
Part 2: Writing the skeleton equationStep 1: Use the determined formulas for the reactants and plug them into the equation. We are told that methane burns in oxygen -- hinting at a combustion reaction. Therefore, we may infer that these are the reactants that yield the products.
Skeleton equations are written with the reactant(s) on the left -- if there are several, they are separated by an addition symbol (+).
With this information, we may begin our equation: \(CH_4 + O_2\), where \(CH_4\) is methane and \(O_2\) is the diatomic molecule of oxygen.
Step 2: Use the determined formulas for the products and plug them into the equation. We are told that the methane burns in oxygen to produce carbon dioxide and water. Hence, we can separate these two as we did with the reactants.
Now, our products side of the reaction will look like this: \(--> CO_2 + H_2O\), where \(CO_2\) is carbon dioxide and \(H_2O\) is water.
Step 3: Write the final equation. All you must do after determining both sides of the equation is simply push them together. Place the reactant side of the equation on the left and the product side of the equation on the right.
This gives us our final equation, \(\boxed{CH_4 + O_2 --> CO_2 + H_2O}\).
Because the problem asks for the unbalanced equation, we do not need to take any further steps of balancing the equation.
what is the cone of uncertainty
Answers
Answer:
The cone of uncertainty represents the evolution of the amount of best-case uncertainty during a project. Predicting the success of a project is extremely difficult. Many factors can influence a company's estimate and contribute to project uncertainties. I hope that this helps you UwU
IM GIVING BRAINLIST HELP Deep, cold water is the __________. Warm, shallow water is the ___________.
Question 3 options:
least dense; densest
densest; least dense
densest; densest
least dense; least dense
Answers
This is due to the weight of the water pushing down on the water molecules below in the deep cold water.
Making the warm shallow water, less dense
In an ecosystem, deep, cold water is the densest while warm, shallow water is the least dense.
What is an ecosystem?An ecosystem is defined as a system which consists of all living organisms and the physical components with which the living beings interact. The abiotic and biotic components are linked to each other through nutrient cycles and flow of energy.
Energy enters the ecosystem through the process of photosynthesis .Animals play an important role in transfer of energy as they feed on each other.As a result of this transfer of matter and energy takes place through the system .Living organisms also influence the quantity of biomass present.By decomposition of dead plants and animals by microbes nutrients are released back in to the soil.
Learn more about ecosystem,here:
https://brainly.com/question/1673533
#SPJ6
A student investigated temperature changes during a reaction. Figure 2 shows the apparatus the student usesWhat is the biggest error in this investigation.Suggest two improvements to the apparatus which would increase the accuracy of the experiment.
[3 marks]

Answers
Answer:
it is becuse the students used wrong apparatus
The biggest error in this investigation is the use of inappropriate types of equipment.
The types of equipment used in the given setup are inappropriate. Suggested improvements in the apparatus to increase the accuracy are given below:
1. The use of a polystyrene cup in the laboratory for any reaction is inappropriate. As a slight increase in temperature causes the decomposition of the chemicals in the cup as well.
2. The use of a thermometer stand is a must. Without using the stand, the tip of the thermometer touches the lid of the container, which causes inaccuracies in the result.
Hence, all the suggestions are listed above.
Learn more about changes in temperature here:
https://brainly.com/question/31081480
#SPJ2
1. The chemical formula of caffeine is C8H10N4O2. Determine the percent composition of the molecule.
Show work please
Answers
Answer:
we find the mass for each element in one mole by multiplying the number of atoms in one molecule with the atomic mass
mC=8Ac=8*12=96g
mH=10AH=10*1=10g
mN=4AN=4*14=56g
mO=2AO=2*16=32g
by adding the masses together we find the molar mass of the molecule
M=mC+nH+mN+mO=96+10+56+32=194g/mole
we apply the rule of threes to find the percentage of each element
194g..96gC..10gH...56gN....32gO
100g....a...........b...........c.............d
a=(100*96)/194=49.48%C
b=(100*10)/194=5.19%H
c=(100*56)/194=28.85%N
d=(100*32)/194=16.48%O
Explanation:
help i suck at chemistry

Answers
Answer:
1. Acid - Red
2. Base - Yellow
3. Salt - Yellow if the reaction produces a base
Explanation:
In an acidic medium, methyl orange turns red, while in a basic medium, it turns yellow.
Sodium chloride solution produces sodium hydroxide, NaOH which is a strong base. Using methyl orange as an indicator gives a yellow colour solution for NaOH.
There are acidic, neutral, and basic salts. Sodium chloride (NaCl) produces a base therefore it would turn yellow as well but likely less distinct than the base.
Answer:
Hello methyl orange is a pH indicator that is commonly used.
If you drip methyl orange to an acidic liquid it will give you the color red.
If it turns yellow after you drip it then the liquid should be a base.
And it gives a yellowish color for neutral liquids
But in this case salt (NaOH) has an exceptional situation which turns orange after adding m.o.
There is no logical explanation (at least for high school level) I am afraid that you need to memorize it.
This chard attached below may help you to recognize it
good luck, hope it helped<3!
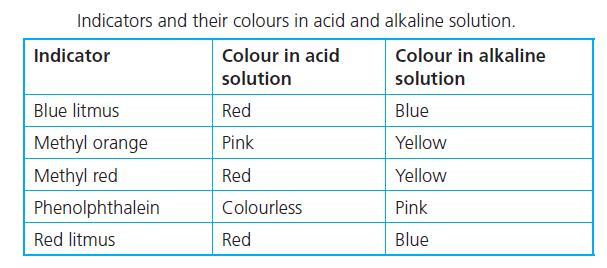
what did immortality-seeking alchemists create by mixing sulfur, charcoal, and potassium nitrate?
Answers
Answer:
Gunpowder was discovered somewhat inadvertently by Chinese alchemists, who were trying to develop an elixir of immortality. If you're not aware, there are three primary components to gunpowder: saltpeter (potassium nitrate), sulfur, and charcoal. ... The use of sulfur by the Chinese is also ancient.
Explanation:
What are the coefficients for the following reaction when it isproperly balanced?
___potassium iodide + ___lead (II) acetate → ___lead (II)iodide +___potassium acetate
Answers
The balanced equation for the reaction between potassium iodide (KI) and lead (II) acetate (Pb(CH₃COO)₂) to form lead (II) iodide (PbI₂) and potassium acetate (CH₃COOK) can be determined by balancing the number of atoms on both sides. Here's how to balance the equation.
To balance the equation, we need to ensure the same number of each type of atom on both sides.First, let's balance the iodine (I) atoms:On the left side, there is one iodine atom in KI, while on the right side, there are two iodine atoms in PbI₂. To balance the iodine atoms, we need to put a coefficient of 2 in front of KI
To know more about iodine visit :
https://brainly.com/question/30957837
#SPJ11
Which statement is the best description of a chemical reaction? A.The combustion of a substance B.The decomposition of particles C.Mixing two substances D.Forming or breaking bonds
Answers
The answer would be D. Forming or breaking bonds.
When forming or breaking bonds, a new substance is created and it’s no longer just a physical change. Sometimes when bonds are breaking and new ones are forming there can be a change in composition.
Hope this helps !
To solve this we must know the concept of chemical reaction. The correct option is option D that is forming or breaking of bonds. There are so many types of chemical reaction reaction like combination reaction, double displacement reaction.
What is chemical reaction?Chemical reaction is a process in which two or more than two molecules collide in right orientation and energy to form a new chemical compound. The mass of the overall reaction should be conserved.
Chemical reaction is a reaction in which the breaking of old bond from the reactant take place while on the product side, formation of new bond take place to form product.
Therefore, the correct option is option D that is Forming or breaking of bonds take place during chemical reaction.
Learn more about the chemical reactions, here:
https://brainly.com/question/3461108
#SPJ5
HELP PLZ!!! Which must be kept in mind when determining if an explanation is correct? whether people reading the explanation agree the information that is on the Internet that there may be more than one way to interpret data whether a person of authority says it is correct
Answers
Answer: C. that there may be more than one way to interpret data
Enge 2020 C is the correct answer
does the hydrogen necessary in the electron transport chain come from the splitting of carbon dioxide molecules
Answers
The hydrogen necessary for this process is ultimately derived from the splitting of carbon dioxide molecules. Yes, the hydrogen necessary for the electron transport chain is derived from the splitting of carbon dioxide molecules in a process known as the Calvin Cycle, or the light-dependent reaction.
In this process, carbon dioxide, water, and light energy are used to create high-energy molecules, such as ATP and NADPH, which are then used in the electron transport chain. During the Calvin cycle, carbon dioxide is reduced by NADPH and ATP to produce a three-carbon molecule called glycerate 3-phosphate.
Hydrogen is removed from glycerate 3-phosphate to create a two-carbon compound known as glyceraldehyde 3-phosphate. This compound is then used to create other compounds, such as glucose, which can be used for energy.
Know more about electron transport chain here:
https://brainly.com/question/24372542
#SPJ11
⚠️⚠️ period and group PLEAAE CHECK OUT THE QUESTIONNS IMAGE ⚠️⚠️

Answers
Answer:
group 5, period 7
How many grams are in .705 moles of Cr?
Answers
Answer:
51.9961 grams
Explanation:
Just did worksheet
What is the total number of electrons in mg
Answers
What is mg????
Explanation
Answer:
12 electrons
Explanation:
For the element magnesium, there are 12 electrons. It means that there are 12 electrons in a magnesium atom.
Hope this helped!