In a certain reaction, you start with 3.0 moles of nitrogen and 5.0 moles of hydrogen. How many moles of ammonia will be produced in the reaction
Answers
The maximum number of moles of ammonia that can be produced is approximately 3.33 moles.
To determine the number of moles of ammonia produced in the reaction, we need to know the balanced chemical equation for the reaction between nitrogen and hydrogen to form ammonia. Assuming it's the Haber process, the balanced equation is as follows:
N₂ + 3H₂ → 2NH₃
From the balanced equation, we can see that 1 mole of nitrogen reacts with 3 moles of hydrogen to produce 2 moles of ammonia.
Given that you start with 3.0 moles of nitrogen and 5.0 moles of hydrogen, we need to determine which reactant is the limiting reactant. The limiting reactant is the one that is completely consumed first, thus limiting the amount of product formed.
Let's calculate the number of moles of ammonia that can be produced from each reactant:
From nitrogen: 3.0 moles N₂ × (2 moles NH₃ / 1 mole N₂) = 6.0 moles NH₃
From hydrogen: 5.0 moles H₂ × (2 moles NH₃ / 3 moles H₂) ≈ 3.33 moles NH₃
Since hydrogen produces fewer moles of ammonia compared to nitrogen, it is the limiting reactant. Therefore, the maximum number of moles of ammonia that can be produced is approximately 3.33 moles.
know more about ammonia here
https://brainly.com/question/12276882#
#SPJ11
Related Questions
Physical vs. Chemical
A physical change is a change that occurs when a substance changes composition forming one or more new substances.
O True
O False
Answers
Answer:
The answer is false.
If this answer was helpful please consider giving brainliest!
Answer:
False
Explanation:
I think it's a chemical change when the composition of a substance changes and forms a new substance
how many moles are there in 4.00 moles of glucose, C₆H₁₂O₆
Answers
2.40 ×10²⁴ molecules are there in 4.00 moles of glucose, C₆H₁₂O₆. A molecule is a collection of at least two atoms.
What is molecule?According on the context, the word can or cannot encompass ions that meet this requirement. A molecule is a collection of at least two atoms bound together by the attractive forces called as chemical bonds.
When speaking of polyatomic ions, the difference between them and ions is frequently ignored in the fields of quantum theory, organic chemistry, especially biochemistry.
number of molecule = number of moles × 6.022×10²³
= 4× 6.022×10²³
= 2.40 ×10²⁴ molecules
Therefore, 2.40 ×10²⁴ molecules are there in 4.00 moles of glucose, C₆H₁₂O₆.
To know more about molecule, here:
https://brainly.com/question/29254782
#SPJ1
how many valence electrons does each atom of arsenic (as) have? arsenic is element 33. it is in period 4 and family 15 (5a or the nitrogen family).
Answers
How many moles of electrons is required to deposit 5.6g of iron from a solution of iron (2) tetraoxosulphate(6)
Answers
Answer:
0.20 mol
Explanation:
Let's consider the reduction of iron from an aqueous solution of iron (II).
Fe²⁺ + 2 e⁻ ⇒ Fe
The molar mass of Fe is 55.85 g/mol. The moles corresponding to 5.6 g of Fe are:
5.6 g × 1 mol/55.85 g = 0.10 mol
2 moles of electrons are required to deposit 1 mole of Fe. The moles of electrons required to deposit 0.10 moles of Fe are
0.10 mol Fe × 2 mol e⁻/1 mol Fe = 0.20 mol e⁻
0.20 mol of electrons is required to deposit 5.6g of iron from a solution of iron (2) tetraoxosulphate(6)
The reduction of iron from an aqueous solution of iron (II).
\(Fe^{+2} +2e^{-} \rightarrow Fe\)
The formula for number of moles is as follows:-
\(Number \ of \ moles=\frac{Mass}{Molar\ mass}\)
The molar mass of Fe is 55.85 g/mol. The moles corresponding to 5.6 g of Fe are:
\(5.6 g \times\frac{1\ mol}{55.85\ g} = 0.10 \ mol\)
2 moles of electrons are required to deposit 1 mole of Fe. The moles of electrons required to deposit 0.10 moles of Fe are:-
\(0.10 mol Fe\times\frac{2\ mol\ e^{-} }{1\ mol\ e^{-}} = 0.20 \ mol e^{-}\)
Hence, 0.20 mol of electrons is required to deposit 5.6g of iron.
To know more about:-
brainly.com/question/12513822
Choose an example of a reaction to which Markovnikov's rule applies.
O CH₂=CH-CH2-CH3 + HBr CH₂ Br=CH2-CH2-CH3
O CH,=CH-CH, CH3 + HBr → CHg =CHBr–CH2–CH3
O CH,=CH-CH,—CH, + HBr → CH,Br–CHBr–CH2–CH, + HBr CH₂Br-CH2-CH2-CH3
O CH₂=CH-CH2-CH3 O CH,=CH-CH2–CH3 + HBr → CH3–CHBr–CH2–CH3
Answers
The example of a reaction to which Markovnikov's rule applies is: CH₂=CH-CH₂-CH₃ + HBr → CH₂Br-CH₂-CH₂-CH₃
In this reaction, the hydrogen atom from HBr adds to the carbon atom with the fewer alkyl substituents (less substituted carbon), while the bromine atom adds to the carbon atom with more alkyl substituents (more substituted carbon). This follows Markovnikov's rule, which states that in the addition of a protic acid (such as HBr) to an asymmetrically substituted alkene, the hydrogen atom adds to the less substituted carbon and the other atom adds to the more substituted carbon.
To learn more about Markovnikov's, https://brainly.com/question/32087294
#SPJ11
theres a diff one of my question
What advancements helped develope the cell theory? (5 points)
a
thermometer
b
flask
c
microscope
d
scale
Answers
Name two test substances that would react with each other to produce water and salt
Answers
Can you help plzzz thank you
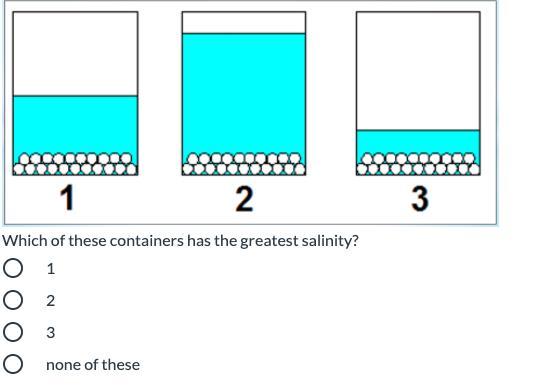
Answers
Answer:
It would be Container 3
Explanation:
Each of the containers has the same amount of salt. Salinity refers to salt level. Since the question is asking for the container with the greatest salinity, you are looking for the container with the least water (because it will be the saltiest out of all of them). Container 3 has the least water.
Hope this helps :)
3 has less water so its saltier
What are the variables for this piston? temperature only temperature and volume pressure and number of molecules volume and number of molecules
Answers
Answer:
Option 2: temperature and volume
Explanation:
took the test
Temperature and volume are the variables for this piston.
What is a temperature?Temperature is a measure of hotness or coldness
The volume of a gas is directly proportional to its absolute temperature. More specifically, for a fixed mass of gas at constant pressure, the volume (V) is directly proportional to the absolute temperature (T).
This is Charles' Law. V = kT, where k is a proportionality constant.
Hence, temperature and volume are the variables for this piston.
Learn more about temperature here:
https://brainly.com/question/11464844
#SPJ2
True or false igneous rocks can only turn into metamorphic
Answers
Answer:
this is false igneous rocks can turn into either sedimentary rocks and they can be metamorphic
Explanation:
i hope this helps your welcome :)
Answer:
False
Explanation:
Igneous rocks can turn into sedimentary rocks as well as metamorphic.
Fluorine, chlorine, and bromine react with gold.
which 1 of the 3 elements will be the most reactive with gold
thanks
Answers
Answer:
gold i believe or possibly bromine
Explanation:
q- how does fluorine react with gold
a- this fluoride compound features gold in its highest known oxidation state. this red solid dissolves in hydrogen fluoride, but these solutions decompose, liberating fluorine.
q- how does chlorine react with gold
a- gold does react with halogens. it'll, for example, react very slowly with chlorine gas at room temperature to form gold chloride, AuCl3. if gold chloride is heated gently, it will decompose to release the pure elements again.
q- how does bromine react with gold
a- gold in bromine solutions dissolves according to electrochemical/chemical (EC) mechanisms. ... in the chemical composition of the mechanism, this monovalent gold bromide disproportionate into gold and stable AuBr −4 , which reports into solution. with respect to pH, there are two characteristic dissolution regions.
** sorry that i can't provide a sure answer**
good luck :)
i hope this helps
have a nice day!
Fluorine will be most reactive with gold.
What is fluorine?
Of almost all of the elements, fluorine seems to be the most electronegative as well as reactive. Fluorine would be a diatomic, pale yellow, extremely corrosive, combustible gas with a strong smell. The lightest halogen would that be. It produces oxygen and even the incredibly corrosive hydrofluoric acid when it combines strongly with water.
What is reactive?Reactivity would be a substance's capacity to chemically combine with several other substances. Iron, for instance, reacts vigorously with oxygen.
As you move down the group, the halogens, which are non-metal elements in Group 7, become less reactive. In contrast to the alkali metals within Group 1 of the particular periodic table, this trend is the opposite. One of the most reactive elements in Group 7 was fluorine.
To know more about fluorine.
https://brainly.com/question/1940697
#SPJ3
Match the following terms describing phase changes with their definitions.
Liquid to gas Solid to gas Solid to liquid Liquid to solid boiling
freezing
melting sublimation
Answers
Liquid to gas - Boiling: The phase change from a liquid to a gas that occurs when the substance reaches its boiling point, resulting in the formation of vapor.
Solid to gas - Sublimation: The phase change from a solid directly to a gas without going through the liquid state.
Solid to liquid - Melting: The phase change from a solid to a liquid when heat is applied, causing the substance to transition from a rigid to a more fluid state.
Liquid to solid - Freezing: The phase change from a liquid to a solid when the substance loses heat, resulting in the formation of a solid crystal lattice.
Therefore, the correct match would be:
Liquid to gas - Boiling
Solid to gas - Sublimation
Solid to liquid - Melting
Liquid to solid - Freezing
To learn more about phase change of matter, visit:
https://brainly.com/question/30720253
#SPJ11
six solutions are made by dissolving a certain amount of each of the six substances in 500. g water. if the freezing point of the each aqueous solution is the same, which substance is added to water in the largest amount, in grams? assume all ionic compounds dissociate 100% to form ions in solution.
Answers
Since KCl has the highest molar mass when it is added to water, it will have the most amount, in grams, if the freezing point of each aqueous solution is the same.
An essential accumulative feature of the solution is a depression in the freezing point. This is signified by: T=Kfm. Here, the symbol for freezing point change is (T). Additionally, m is morality, and Kf is the freezing point constant. One of the matter's physical characteristics is its temperature.
1) Options list:
A. (NH₄)₂CO₃
Ionic Equation:
(NH₄)₂CO₃ → 2(NH₄)⁺ + {CO₃}⁻ [i = 3]
B. KCl
Ionic equation:
KCl → K⁺ Cl⁻ [i = 2]
D.NH₄Cl
Ionic equation:
KCl → K⁺ Cl⁻ [i = 2]
F. NaCl
Ionic equation:
NaCl → Na⁺ + Cl⁻ [i = 2]
G. CH₃OH
Ionic equation:
CH₃OH → CH₃⁺ + OH⁻ [i = 2]
H. MgCl₂
Ionic equation:
MgCl₂ → Mg² + 2Cl⁻ [i = 3]
2) Utilizing the formula
ΔT = iKᵇmsolute
ΔT = iKfmsolute
ΔT = T° - T
Here, T° is both the solution's temperature and the temperature of the pure solvent.
Suppose that T° is zero.
ΔT = 0 - T = -T
The greater negative the temperature, the lower the freezing point. The freezing point of the solution is lowered when a solute is added to the solvent. The formula states that a lower molality gives a solute the ability to be added to a greater extent and produce a smaller change in temperature.
To obtain a smaller shift in temperature, the molality should be the lowest possible. The quantity of moles of solute in one kilogram of solvent is referred to as molality.
Morality = (Number of moles / 1) * (1 / Mass of solvent in kg)
Morality = (Mass of solute / Molar Mass of solute) * (1 / Mass of solvent in kg)
In this case, the solvent weighs 0.5 kg (constant)
Morality ∝ (Mass of solute / 1)
OR
Morality ∝ (1 / Molar Mass of solute)
With a smaller mass of a material or a larger molar mass of a solute, the molality will be lower. Only the solvent's freezing point and the solute's molar mass are under control under the given conditions. Despite the fact that KCl has a higher molar mass than (NH₄)₂CO₃, (NH₄)₂CO₃ will produce more ions at 100% dissociation than KCl. As a result, there will be fewer moles for the KCl.
The amount of KCl in grams will be the highest since it has the highest molar mass when it is added to water.
To learn more about Freezing point, refer to this link:
https://brainly.com/question/29312879
#SPJ4
COMPLETE QUESTION:
Six solutions are made by dissolving a certain amount of each of the six substances in 500. g water. If the freezing point of each aqueous solution is the same, which substance is added to water in the largest amount, in grams? Assume all ionic compounds dissociate 100% to form ions in the solution.
ΔT = iKbmsolute ΔT = –iKfmsolute
Select one:
A. (NH4)2CO3
B. KCl
C. The largest mass (g) of substance added is the same for two of the solutions.
D.NH4Cl
E. Insufficient information is provided.
F. NaCl
G. CH3OH
H. MgCl2
The correct answer should be "B" or KCl, but I don't understand why
Polarities of analyte functional group increase in the order of hydrocarbon ethers < esters
Answers
The correct order of the increasing polarity of the analyte functional group isEthers < Esters.
The given statement is "Polarities of analyte functional group increase in the order of hydrocarbon ethers < esters." The order of polarities of functional groups is the order of their increasing polarity (i.e., less polar to more polar) based on their electron-donating or withdrawing ability from the rest of the molecule.Polarity of analyte: The analyte's polarity is directly proportional to the dipole moment of the functional group, which is associated with a difference in electronegativity between the atoms that make up the functional group.The electronegativity of an element is its ability to attract electrons towards itself. The greater the difference in electronegativity between two atoms, the more polar their bond, and hence the greater the polarity of the molecule.
To find the correct order of the increasing polarity of the analyte functional group, let's first compare the two groups: hydrocarbon ethers and esters. Here, esters have a carbonyl group while ethers have an oxygen atom with two alkyl or aryl groups. The carbonyl group has more electronegative oxygen, which pulls electrons away from the carbon atom, resulting in a polar molecule. On the other hand, ethers have a less polar oxygen atom with two alkyl or aryl groups, making them less polar than esters. Therefore, the correct order of the increasing polarity of the analyte functional group isEthers < Esters.
To know more about polarity visit:-
https://brainly.com/question/33242453
#SPJ11
LESSON 1
Content Practice B
1
Position and Motion
Directions Complete these purphs by writing the correct terms on the lines. Some terms might be used more
you must first choose ain)
To describe an object's (1. )
(2. )
as a starting place. From there, you must specify the
in
(3. )
to the object and the (4. )
which it lies from the starting place. If you are giving directions to two objects located
it can sometimes
in different directions from the same (5. )
direction
be helpful to describe one object as being in the (6. )
direction
from that place and the other in the (7. )
An object is in (8. )
any time its
is changing. In most cases, such a change involves changes in
(10. )
and (11. )
from the starting
point. However, if an object returns to its starting point, its
(12. )
is zero, even though it might have traveled
Answers
Answer:
1. Motion
2. Position
3. Relation
4. Distance
5. Reference point
6. North
7. South
8. motion
9. Speed
10. Distance
11. Direction
12. displacement
(Please could you kindly mark my answer as brainliest you could also follow me so that you could easily reach out to me for any other questions)
What is the opposite force to pushing off the ground to jump?
Answers
Answer:
Newton's Third Law of Motion states that to every action there is an equal and opposite reaction. When the earth pushes on you to send you into the air after jumping, you also push on the earth with the same force. But forces are different from accelerations. A force F is a push or pull on an object
Explanation:
Answer:
Friction
Explanation:
Friction causes an opposite force
When the volume of a gas is
changed from m3 to 15.5 m3
the temperature will change from
159 K to 456 K.
Assume that the number of moles and the
pressure remain constant.

Answers
Charles law states that there is a directly proportional relationship between the volume and the temperature of the gas at certain pressure.
Therefore,
V1/T1 = V2/T2
V1 is unknown
T1 = 159K
V2 = 15.5m3
T2 = 456K
V1/159 = 15.5/456
V1 = (15.5*159)/456 = 5.404m3.
When the volume of a gas is changed from 5.404 m3 to 15.5 m3 the temperature will change from 159 K to 456 K.
Identifying Parts of a Chemical Reaction

Answers
Given chemical reaction,
Glucose + Oxygen → carbon dioxide + water
Reactants : Glucose and oxygen.
Products : carbon dioxide and water.
Parts of chemical reaction:Each chemical reaction is made up of three main components: the reactants (located on the left side of the reaction equation), the products (located on the right side of the reaction equation), and the particular reaction conditions, which are listed above or below the arrows in the middle of the reaction equation.
Combustion is one of the five fundamental types of chemical reactions, along with combination, decomposition, single-replacement, and double-replacement. You can classify a reaction into one of these groups by looking at the reactants and products of the reaction in question. It is possible for some reactions to fall into more than one category.
To know more about chemical reaction visit:
https://brainly.com/question/29039149
#SPJ1
A compound contains only aluminum and carbon. 0.03 moles of this compound reacted with excess water to form 0.12 moles of AI(OH)3 and 0.09 moles of CH4 write a balanced equation for this reaction.
Answers
Explanation:
4Al+3C+H2O→4Al(OH)3+3CH4
Balanced reaction:- 4Al+3C+HH O→4Al(OH)3+3CH4
A solution of NaCl was prepared in the following manner: 0.0842 g of NaCl is massed out on an analytical balance. The solid is transferred to a 25.00 mL volumetric flask. Deionized water is added to the flask such that the bottom of the meniscus is at the line. A 1.00 mL aliquot of the stock solution is transferred to a 50.00 mL volumetric flask using a volumetric pipet and diluted to volume. 6. Calculate the concentration of NaCl in the resulting solution in mg/L NaCl. (answer = 67.4 mg/L) 7. Calculate the concentration of NaCl in the resulting solution using propagation of error through the calculation. Use the manufacturer's tolerance values as the absolute error. The tolerances can be found in Chapter 2 of the Harris text. Assume a Class 1 balance and Class A glassware. Treat the tolerances as random error. (answer = 67.4+0.4 mg/L) 8. Identify 2 possible sources of random (indeterminate) error. Identify 2 possible sourses of systematic (determinate) error.
Answers
Two possible sources of systematic (determinate) error in the experiment are; Incorrect calibration of volumetric glasswareIncorrect mass of NaCl
To calculate the concentration of NaCl in the resulting solution in mg/L NaCl, we can use the formula; Concentration (mg/L) = (Mass of solute ÷ Volume of solution in L) × 1000 g / 1 mg NaCl is present in the stock solution of 25 mL. So, the mass of NaCl in the solution would be;0.0842 g ÷ 25 mL = 0.00337 g/mL. Now, in the resulting solution, a 1.00 mL aliquot of the stock solution is transferred to a 50.00 mL volumetric flask and diluted to volume. Therefore, the volume of the resulting solution is 50.00 mL. We will substitute these values in the formula, Concentration (mg/L) = (0.00337 g/mL ÷ 50 mL) × 1000 g / 1 mg concentration (mg/L) = 67.4 mg/L. Therefore, the concentration of NaCl in the resulting solution in mg/L NaCl is 67.4 mg/L.7. Concentration = 67.4 mg/LTolerance = 4.28 mg/LTotal concentration = 67.4 + 4.28 mg/L = 71.68 mg/LWe round off this value to one decimal place; Total concentration = 71.7 mg/LTherefore, the concentration of NaCl in the resulting solution using propagation of error through the calculation is 67.4+0.4 mg/L.8. Two possible sources of random (indeterminate) error in the experiment are; Errors in temperature measurement. Errors in measurement of water volume. Two possible sources of systematic (determinate) error in the experiment are; Incorrect calibration of volumetric glasswareIncorrect mass of NaCl.
Learn more about NaCl
https://brainly.com/question/32275922?
#SPJ11
The label CORROSIVE on a chemical container indicates
Answers
The label CORROSIVE on a chemical container indicates that the contents are corrosive, meaning that contact with these materials can cause damage to living tissue and materials.
It is important to exercise caution when handling these materials and to ensure that the containers are well labeled and stored in a safe and secure location.
Corrosive chemicals are substances that can cause damage or destruction to other substances with which they come into contact by means of a chemical reaction. These reactions can occur on contact, or over a period of time. Corrosive chemicals can cause damage to the skin, eyes, respiratory tract, and other parts of the body.
Learn more about chemical container
https://brainly.com/question/29488607
#SPJ4
If a 67.3G rock is dissolved in 2.00L of acid, what is the molar concentration of gold in the acid solution
Answers
Answer:
[Au] = 0.171 M
Explanation:
For this question, we assume the rock is 100 % gold.
First of all, we determine the moles of gold
67.3 g . 1mol/ 196.97g = 0.342 moles
Molar concentration is defined as the moles of solute, contained in 1L of solution.
Our solution volume is 2L.
M = 0.342 mol / 2L = 0.171
Molar concentration, also called molarity of solution is the most typical unit of concentration.
calculate the mass of 25,000 molecules of nitrogen gas. (1 mole = 6.02 × 1023 molecules) group of answer choices 7.00 × 105 g 5.81 × 10−19 g 5.38 × 1026 g 1.16 × 10−18 g
Answers
The mass of the 25,000 molecules of Nitrogen gas is found to be 0.0000017 g (1.7 x 10⁻⁶ g).
We must use the molar mass of nitrogen and the Avogadro's number to get the mass of 25,000 molecules of nitrogen gas.
Nitrogen (N₂) has a molar mass of 28 g/mol.
The number of particles in one mole of any substance determined by Avogadro is 6.02 x 10²³.
One nitrogen gas molecule's mass can be determined as follows:
Nitrogen's (N₂) molecular weight is 28 g/mol.
Nitrogen gas molecules total 25,000.
Nitrogen gas moles equal (25,000 molecules) / (6.02 x 10²³ molecules/mol)
(Num. of moles) x = mass of nitrogen gas (molar mass)
[(25,000 molecules) / (6.02 x 10²³ molecules/mol)] is a formula for the mass of nitrogen gas. x (28 g/mol)
Nitrogen gas mass = 0.00000117 g
Therefore, 25,000 nitrogen gas molecules have a mass of about 0.00000117 g.
To know more about Avogadro number, visit,
https://brainly.com/question/859564
#SPJ4
Determine the molar solubility of some salt with the generic formula AB_2 if K_sp = 2.56 x 10^2. O 4 M O 1 M O 10 M O 0.1 M
Answers
The molar solubility of the salt AB_2 is 4.06 M.
To determine the molar solubility of a salt with the formula AB_2 and a given K_sp value, we need to use the following equilibrium expression:
K_sp = [A^2][B]^2
where [A] and [B] represent the molar concentrations of the ions A^+ and B^- in solution.
Let's denote the molar solubility of the salt AB_2 as x. Then, at equilibrium, the concentrations of A^+ and B^- ions will be equal to 2x and x, respectively, because the salt dissociates into two A^+ ions and one B^- ion. Substituting these values into the equilibrium expression, we get:
K_sp = (2x)^2 * x = 4x^3
Now we can solve for x:
x = (K_sp/4)^(1/3)
Plugging in the given value of K_sp = 2.56 x 10^2, we get:
x = (2.56 x 10^2/4)^(1/3) = 4.06 M
Therefore, the molar solubility of the salt AB_2 is 4.06 M.
Click the below link, to learn more about Molar solubility of salt:
https://brainly.com/question/29357702?referrer=searchResults
#SPJ11
0.32 moles of oxygen gas has a temperature of 27°C and pressure of 2 atm in a closed container. What is the volume?
Answers
Answer:
3.9 L
First convert temperature to Kelvin
then use the ideal gas law
use algebra to solve for V
Since your solving for volume, your answer should be in Liters.

Which two statements describe a mixture?
O A. It is a pure substance.
O B. It includes atoms of different elements.
O C. Its parts can be separated using physical means.
O D. It can result from chemical bonding.
Answers
Answer:
d
Explanation:
because the chemicals or substances mix together so it's basically like chemical bonding.
ammonia gas reacts with oxygen gas, o2 (g), to produce nitrogen dioxide and water. when 32.4 g of ammonia gas reacts with 87.3 g of oxygen gas to produce 64.5 g of nitrogen dioxide, what is the percent yield for the reaction?
Answers
the percent yield for the reaction = 83.5086
The substance(s) that are initially a part of a chemical reaction are referred to as reactants or reagents. A chemical change often characterizes chemical reaction, and they produce one or more products that typically have characteristics distinct from the reactants. The so-called elementary reactions are reactions that frequently consist of a series of discrete steps, and the information regarding the specific sequence of events is contained within the reaction mechanism. Chemical equations are used to describe chemical processes. These equations represent the initial substances, final products, and occasionally intermediate products as well as the reaction circumstances symbolically.
When a temperature and chemical concentration are known, chemical reaction proceed at a predictable rate. As temperature rises, reaction rates often increase as more thermal energy is available to reach the activation energy.
Learn more about the reaction here:
https://brainly.com/question/14025220
#SPJ4
What is a polysaccharide and what are the differences between the plant polysaccharides?
Answers
Polysaccharide is a type of carbohydrate (such as glycogen, cellulose, or starch) whose molecules are made up of many sugar molecules bound together.
What purpose does the polysaccharide serve?
In general, polysaccharides serve one of two purposes: they either store energy or sustain structural integrity. Highly compact polymers like starch and glycogen are employed to store energy. In plants and animals, cellulose and chitin, two linear polymers, provide structural support.
What is plant polysaccharide?
More than half of the carbs we consume come from starch, which is the most significant source of carbohydrates in the human diet. Granules of it can be found in plants. Amylose and amylopectin, two polymers, are combined to form starch. 10%–30% amylose and 70%–90% amylopectin make up natural starches.
Learn more about the Polysaccharides with the help of the given link:
https://brainly.com/question/780562
#SPJ4
How many grams of H 2 O can be produced with 6.3 moles of O 2 ?
Answers
Answer:
3.15×18
56.7
................
Copy the sentences below. Correcting the five mistakes.
In a displacement reaction, a less reactive metal pushes out a more reactive metal from its compound. For example, iron displaces aluminium from aluminium oxide.
Answers
Answer:
In a displacement reaction, a more reactive metal pushes out a less reactive metal from its compound. For example, aluminium displaces iron from iron oxide.
Explanation:
Because aluminium is more reactive than iron, it displaces iron from iron(III) oxide. The aluminium removes oxygen from the iron(III) oxide: iron is reduced.
...
Hope this answer can help you. Have a nice day!
In a displacement reaction, a more reactive metal pushes out a less reactive metal from its compound. For example, aluminium displaces iron from iron oxide.
What is a compound?Compound is defined as a chemical substance made up of identical molecules containing atoms from more than one type of chemical element.
Molecule consisting atoms of only one element is not called compound.It is transformed into new substances during chemical reactions. There are four major types of compounds depending on chemical bonding present in them.They are:
1)Molecular compounds where in atoms are joined by covalent bonds.
2) ionic compounds where atoms are joined by ionic bond.
3)Inter-metallic compounds where atoms are held by metallic bonds
4) co-ordination complexes where atoms are held by co-ordinate bonds.
They have a unique chemical structure held together by chemical bonds Compounds have different properties as those of elements because when a compound is formed the properties of the substance are totally altered.
Learn more about compound,here:
https://brainly.com/question/13516179
#SPJ2