Help answer fast please What is an example of homestasis?
A. You drink a bunch of redbull and your heart rate goes up
B. You run, your heart rate goes up, and then your heart rate goes back to its resting rate
C. A person has a fever
D. A person is thirsty but does not drink water
Answers
Answer:
c maybe sorry if its wrong
Explanation:
Related Questions
Give a real-world example of an energy transformation that uses two of the following forms of energy: chemical, mechanical, nuclear, gravitational, radiant, electrical, thermal (heat), and/or sound.
Answers
Answer:
Nuclear energy gets converted into heat energy during a detonation and is also used in nuclear power plants where the heat energy form the nuclear energy gets converted into mechanical energy by converting water into steam, rotating the turbines and converting that into electrical energy
Explanation:
Or heat engines, such as the internal combustion engine used in cars, or the steam engine. (Heat - Mechanical energy)
which ion (cation or anion) remained the same between sodium sulfate and sodium chloride?
Answers
Answer:
Sodium (Na) is a cation, which means it has a positive charge.
Sodium forms ionic compounds with different anions, such as sulfate (SO4 2-) and chloride (Cl-).
In sodium sulfate (Na2SO4), sodium is still a cation with a charge of +1, while sulfate is an anion with a charge of -2.
In sodium chloride (NaCl), sodium is still a cation with a charge of +1, while chloride is an anion with a charge of -1.
Therefore, the cation that remained the same between sodium sulfate and sodium chloride is sodium (Na+).
Explanation:
Sodium (Na) is a cation, which means it has a positive charge.
Sodium forms ionic compounds with different anions, such as sulfate (SO4 2-) and chloride (Cl-).
In sodium sulfate (Na2SO4), sodium is still a cation with a charge of +1, while sulfate is an anion with a charge of -2.
In sodium chloride (NaCl), sodium is still a cation with a charge of +1, while chloride is an anion with a charge of -1.
Therefore, the cation that remained the same between sodium sulfate and sodium chloride is sodium (Na+). In chemistry, there are two types of ions: cations and anions.
Cations are ions that have a positive charge because they have lost one or more electrons. Anions are ions that have a negative charge because they have gained one or more electrons.
Sodium (Na) is a cation with a charge of +1, meaning it has lost one electron. In both sodium sulfate (Na2SO4) and sodium chloride (NaCl), sodium is still a cation with a charge of +1.
Therefore, the cation that remained the same between sodium sulfate and sodium chloride is sodium (Na+).
The cation (Na⁺) stayed the same in both cases, whereas the anions (SO₄²⁻ and Cl⁻) differed. The cation (Na+) remained the same in both cases, whereas the anions (SO₄²⁻ and Cl⁻) differed.
The ion that remained the same between sodium sulfate and sodium chloride is cation. An ion is an atom that has lost or gained one or more electrons. A positive ion is known as a cation since it has lost one or more electrons, whereas a negative ion is known as an anion since it has gained one or more electrons. The ions are crucial for the chemical reactions to occur and salt formation.
In sodium sulfate (Na2SO4), the sodium (Na) atom gives away two electrons to create Na+. In this example, the Na+ ion is formed, which is a cation. In sodium chloride (NaCl), the sodium (Na) atom gives away one electron to create Na+. In this example, the Na+ ion is also formed, which is a cation.
The cation (Na+) stayed the same in both cases, whereas the anions (SO₄²⁻ and Cl⁻) differed. As a result, the cation (Na+) remained the same in both cases, whereas the anions (SO₄²⁻ and Cl⁻) differed.
To learn more about cation check the link below-
https://brainly.com/question/14309645
#SPJ11
helium is a gas at the boiling point of liquid nitrogen, 77.0 k. if 2.00 g of he is placed inside a 1.50 l container immersed in liquid nitrogen at 77.0 k, what is the pressure exerted by the helium gas?
Answers
The ideal pressure exerted by the helium gas is 426580 Pa.
We need to know about the ideal gas theory to solve this problem. The ideal gas is assumed that there is no interaction between particles in a gas. It can be determined by the equation
P . V = n . R . T
where P is pressure, V is volume, n is the number of moles gas, R is the ideal gas constant (8.31 J/mol.K) and T is temperature.
From the question above, we know that the parameters of Helium gas are
T = 77 K
m = 2 g
V = 1.5 L = 1.5 x 10¯³ m³
Mr He = 2
By substituting the given parameters, we can calculate the pressure
P . V = n . R . T
P . 1.5 x 10¯³ = 2 / 2 . 8.31 . 77
P = 426580 Pa
Find more on ideal pressure at: https://brainly.com/question/25290815
#SPJ4
Do metals become more reactive as you move from left to right on the periodic table?.
Answers
The periodic table does not show that metals become more reactive as you move from left to right. However, the reactivity drops off from the periodic table's left to right.
Additionally, the periodic table is read left to right. Because an element must have high energies to remove the additional electrons from their valence shells, elements have lesser chemical reactivity. A tabular representation of the chemical elements is known as the periodic table, or periodic table of the (chemical) elements and reactivity drops off from the periodic table's left to right. It is commonly regarded as an icon of chemistry and is utilized extensively in physics, chemistry, and other sciences. A periodic table is a list of chemical elements ordered in order of atomic number, typically in rows.
Learn more about periodic table here
https://brainly.com/question/11155928
#SPJ4
Can someone help me answer this question pls
“Apakah cara anda mengenal pasti gas oksigen dan gas karbon dioksida?”
Answers
Answer:
There are several ways to identify oxygen and carbon dioxide gases. Here are a few possible methods:
Visual observation: Oxygen is a colorless, odorless gas, while carbon dioxide is a colorless gas with a slightly sour smell.Chemical reactions: Oxygen is highly reactive and can support combustion, while carbon dioxide does not burn.Testing with a gas detector: A gas detector can be used to detect and measure the concentration of gases in the air. Oxygen and carbon dioxide can be identified using specific sensors in the gas detector.Lab testing: Oxygen and carbon dioxide can be analyzed in a laboratory using specialized equipment, such as a gas chromatograph, to identify and quantify the gases.Physical properties: Oxygen and carbon dioxide have different physical properties, such as boiling point, density, and solubility in water, which can be used to distinguish between the two gases.use these diagrams to explore the differences between these two processes to breakdown ozone in the questions below. q3.10.2 points grading comment: is there a relationship between the number of energy barriers and the number of steps in the reaction?
Answers
In the diagrams provided, there are two processes shown for breaking down ozone. The first process involves a single-step reaction, while the second process involves a multi-step reaction with two energy barriers.
In the single-step reaction, a molecule of ozone is directly converted into oxygen and an oxygen radical. This process has only one energy barrier, which means that it requires less energy to occur.
In the multi-step reaction, the breakdown of ozone occurs in two steps, with the formation of an intermediate molecule in between. The first step requires energy to break the ozone molecule into an oxygen molecule and an oxygen radical. The intermediate molecule is then formed when the oxygen radical reacts with another ozone molecule. The second step requires energy to break down the intermediate molecule into two oxygen molecules. This process has two energy barriers, which means that it requires more energy to occur.
Therefore, we can conclude that there is a relationship between the number of energy barriers and the number of steps in the reaction. The more steps a reaction has, the more energy barriers it will have to overcome. This also means that the reaction will require more energy to occur. In the case of breaking down ozone, the single-step reaction is more energetically favorable than the multi-step reaction with two energy barriers.
To know more about ozone visit:
https://brainly.com/question/27911475
#SPJ11
giúp mìnhh với mọi người
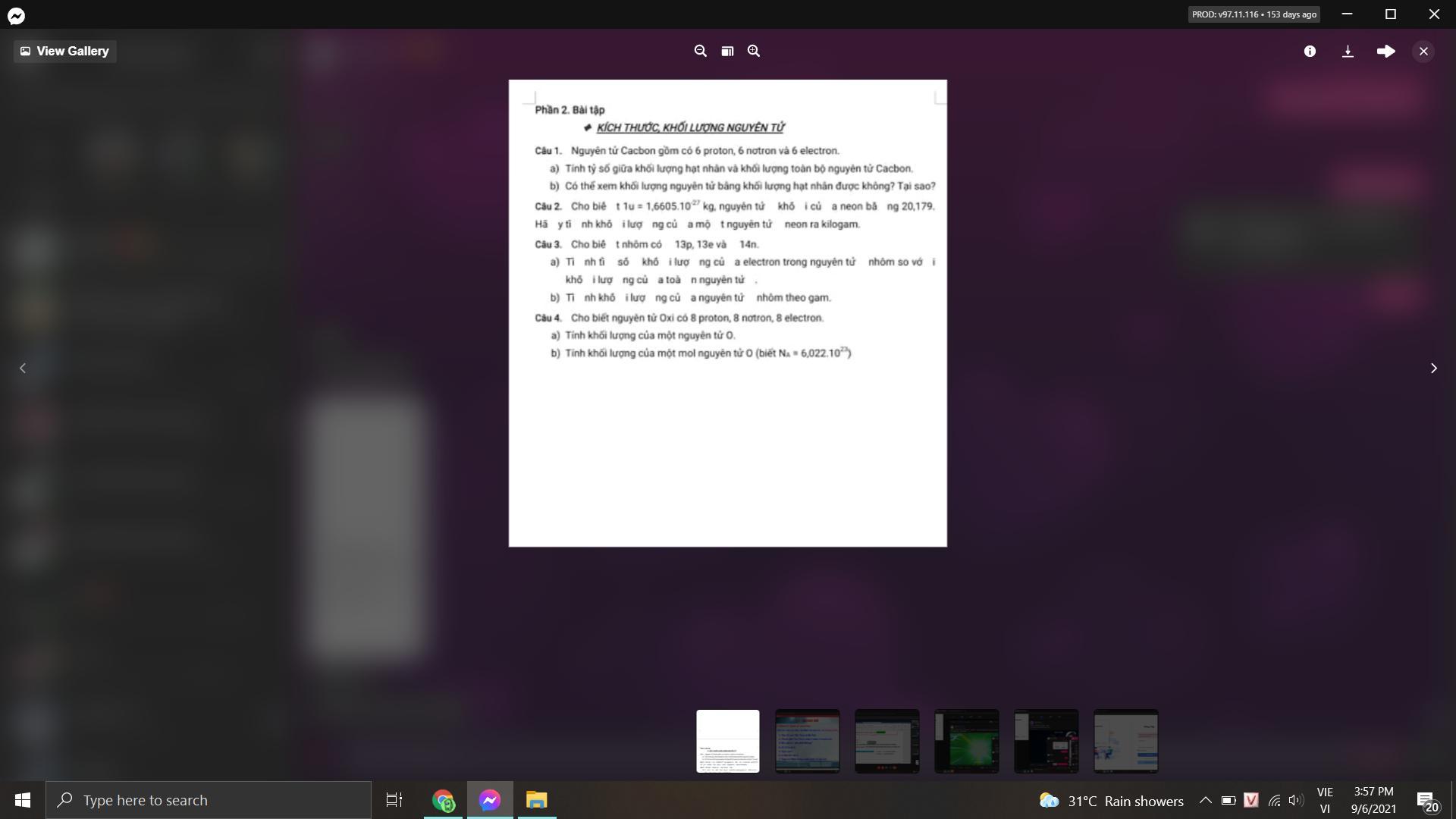
Answers
Answer:ur need to gorw up
Explanation:y seroiuslyi
Of the following, which element has the highest first ionization energy?
Rb
Na
Ca
Sr
Answers
Rb (rubidium) has the highest first ionization energy of the elements listed. This is because Rb has the highest atomic number (37) out of the elements given. The higher the atomic number of an element, the higher its ionization energy.
What is atomic number?Atomic number is a number that represents the number of protons in an atom's nucleus. It is also referred to as the proton number and is usually denoted by the symbol Z. The atomic number uniquely identifies a chemical element and is identical to the charge number of the nucleus. The atomic number of an element can range from 1 (hydrogen) to as high as 118 (ununoctium), and each element has a unique atomic number. For example, carbon has an atomic number of 6, oxygen has an atomic number of 8, and helium has an atomic number of 2. The periodic table of elements is arranged according to the atomic number of each element, which allows for an easy comparison of elements and their properties.
To learn more about atomic number
https://brainly.com/question/11353462
#SPJ4
according to the uncertainty principle, as you localize a wave in time, you become more uncertain about its:_____.
Answers
Answer:
position
Explanation:
cant know both at the same time
What intermolecular forces are in solid NaCl salt?
Answers
The intermolecular forces are in the solid NaCl salt is ion - ion forces or the dipole - dipole forces of the interaction.
The Na is the metal and is capable of the donating the electrons and the Cl is the non metal and have capability of accepting the electrons. This makes the Na to form the cation and the Cl to make the anion. The dipole - dipole forces are present in the molecule which contains the oppositely charged ions one is positively charged ion and the other is the negatively charged ion.
Thus , the NaCl solid has the ion - ion intermolecular forces of the interaction .
To learn more about intermolecular force here
https://brainly.com/question/30542287
#SPJ4
A sample of oxygen gas has a pressure of 6.58 kPa at 539 K. If the volume does not change, what will be the pressure at -62.0°C?
Answers
Given that the volume remains constant, the sample of oxygen gas will have a pressure of 10.67 kPa at a temperature of -62.0°C, according to the question.
What is pressure?The force per unit area applied to a surface is described by the fundamental physical quantity known as pressure. It is quantified in units of force like pounds per square inch (psi) or newtons per square metre (N/\(m^2\)). In addition to measuring the amount of force delivered to an area, pressure can also be used to gauge how much work a system has accomplished.
Because a gas sample's pressure and temperature are inversely correlated, when the temperature varies, so does the gas pressure. The Ideal Gas Law, which states that P*V = n*R*T, where P is pressure, V is volume, n is the number of moles, R is the ideal gas constant, and T is temperature, describes the relationship between pressure and temperature for a certain volume of gas.
As a result, we may modify the Ideal Gas Law equation to solve for P in order to determine the pressure at -62.0°C:
P = (n*R*T) / V
P = (n*R*(-62.0 + 273.15)) / V
P = (n*R*211.15) / V
P = (6.58 kPa * 8.314 J/K·mol * 211.15 K) / V
P = 10.67 kPa
Given that the volume remains constant, the pressure of the sample of oxygen gas will be 10.67 kPa at -62.0°C.
To learn more about pressure
brainly.com/question/24719118
#SPJ1
16.Sulfur has 16 electrons. After it bonds with Magnesium it acquires a 2- charge. How many electrons does the S2- ion contain?Select one:a. 2b. 8c. 16d. 18
Answers
18 electrons.
Explanations:According to the question, we have that sulphur has 16 electrons and after bonding with magnesium acquired a 2-charge. The sulphur ion S2- shows that sulphur has gained 2electrons to become stable.
Amount of electron S2- have = 16 electron + 2electrons
Amount of electron S2- have = 18 electrons
Hence the number of electrons S2- ion have is 18 electrons.
True or False: PERIODS on the periodic table run up and down.
Answers
Answer:
True
Explanation:
I guessed XD But I also used Google and that's what I got
In aqueous solution, classify these compounds as strong acids, weak acids, strong bases, weak bases, or other.
Strong Acid Weak Acid Strong Base Weak Base Other
CH3COOH NaCI HNO3 H2CO3 LIOH (CH3)3N HCI NH3 HF Ba(OH)2
Answers
Strong Acid: HNO3 and HCI Weak Acid: CH3COOH and H2CO3 Strong Base: LIOH and Ba(OH)2Weak Base: NH3Other: NaCI and (CH3)3N
A substance that produces more hydrogen ions in an aqueous solution is called a strong acid, while one that produces fewer hydrogen ions is called a weak acid. When it comes to strong acids, they are only partially ionized, whereas weak acids are only partially ionized.
Strong Acid: HNO3 and HCI Strong acids are compounds that ionize 100 percent when they are dissolved in water, resulting in a large concentration of hydrogen ions (H+). As a result, they have a low pH, which can cause severe chemical reactions when they come into touch with skin and other materials.
Weak Acid: CH3COOH and H2CO3Weak acids are substances that only partially ionize in water, resulting in a small concentration of hydrogen ions (H+). These acids have a higher pH than strong acids, making them less hazardous than strong acids. When they react with bases or alkaline materials, they produce salt and water.
Strong Base: LIOH and Ba(OH)2Strong bases are compounds that ionize 100 percent when dissolved in water, resulting in a high concentration of hydroxide ions (OH-). Strong bases, like strong acids, have a pH of less than 7. They are extremely caustic and can be harmful to the skin.
Weak Base: NH3A weak base is a compound that only partially ionizes in water, resulting in a small concentration of hydroxide ions (OH-). They have a pH greater than 7. Weak bases are less hazardous than strong bases because they produce less OH- ions, which can damage the skin.
Other: NaCI and (CH3)3NNaCl is not a base or an acid because it does not ionize in water. It is a neutral compound. (CH3)3N is a weak organic base. In water, it only partially ionizes. Its main function is to capture hydrogen ions.
To know more about Acid visit :
https://brainly.com/question/29796621
#SPJ11
How does the radius of the central atom affect the boiling points of Group 16 halides?
Answers
Show a numerical setup for converting 120. kPa to atmospheres.
Answers
Answer and Explanation:
To convert 120 kPa to atmospheres, we can use the following conversion factor:
1 atmosphere = 101.325 kPa
We can use this conversion factor to set up a proportion and solve for the number of atmospheres.
120 kPa * (1 atm / 101.325 kPa) = 1.184 atm
Therefore, 120 kPa is equivalent to approximately 1.184 atmospheres (rounded to three decimal places).
Find the kinetic energy of a 5kg bowling ball that is traveling 2 meters per second
Answers
Explanation:
KE = ½ mv²
= ½ x 5 x 2² joule
= 10 joule
How can you tell if an atom is an isotope?
Answers
Answer:
Look up at the atom on the periodic table of elements and find out what its atomic mass is. Subtract the number of protons from the atomic mass. This is the number of neutrons that the regular version of the atom has. If the number of neutrons in the given atom is different, than it is an isotope.
Hydrogen peroxide is placed in sunlight and reacts slowly to form oxygen and water.
a. Rewrite the chemical reaction as a word equation
b. State the evidence that a chemical reaction has occurred
c. Is the reaction exothermic or endothermic? Explain your reasoning
Answers
a. The chemical reaction can be rewritten as a word equation as follows:
Hydrogen Peroxide -> Oxygen + Water
b. Evidence that a chemical reaction has occurred includes the production of a new substance (oxygen and water), a change in the physical properties of the reactants (change in color, odor, or temperature), and the release of energy (evolution of oxygen gas).
c. The reaction is likely exothermic, which means it releases energy. This is because the reaction produces new substances (oxygen and water) and releases energy in the form of oxygen gas. In an endothermic reaction, the reactants would absorb energy from the surroundings.
phcl2 lewis structure
Answers
Answer:
What do you want answered?
Explanation:
draw a single lewis structure for each of the following molecules and ions. which has two lone pairs on the central atom? group of answer choices of2 sf2 all of these h2o
Answers
A Lewis structure is a representation of a molecule or ion that shows the arrangement of atoms and valence electrons. Among the given molecules and ions, both OF2 (oxygen difluoride) and H2O (water) have two lone pairs on the central atom.
In the Lewis structures provided, OF2 consists of an oxygen atom bonded to two fluorine atoms. The oxygen atom in OF2 has two pairs of non-bonding electrons (lone pairs) around it, making a total of two lone pairs on the central atom. It is a covalent bond. It can be seen in image 1.
In contrast, SF2 consists of a sulfur atom bonded to two fluorine atoms. The sulfur atom in SF2 has only one pair of non-bonding electrons (lone pair) around it, so it does not have two lone pairs on the central atom. It is a covalent bond. It can be seen in image 2.
On the other hand, H2O contains two hydrogen atoms bonded to an oxygen atom. The oxygen atom in H2O also has two lone pairs of electrons, resulting in a total of two lone pairs on the central atom. It is a covalent bond. It can be seen in image 3.
Learn more about lone pairs here:
https://brainly.com/question/31961394
#SPJ11



1s22s22p63s23p6
How many unpaired electrons are in the atom represented by the electron configuration above?
a)0
b)1
c)2
d)3
Answers
Answer:
I am working on science rn 2
Purchase process: (50) The process begins with a department admin sending a purchase request to the IT department. The IT Manager reviews the request and if approved, requests a quote from Apple, Dell, HP, ASUS and Lenovo. If rejected, the request is sent back to the admin for review and has to be resubmitted to the IT Manager. The best price will be sent to the admin and once approved, the IT manager finalizes the vendor and then prepares the purchase request. The Procurement Supervisor receives the request and issues the purchase order to the vendor. The Procurement Supervisor then reviews the invoice and processing time from the vendor. By the end of the processing time, if the tracking number was not received, the Supervisor cancels the order. If vendor provides the tracking number, Procurement Supervisor collects the product once delivered and simultaneously submits the payment. Once both the steps are done, the process ends as the purchase is completed.
Answers
The purchase process involves steps such as initiating a request, vendor selection, approval, purchase order issuance, product delivery, and payment, ensuring a systematic approach to procurement for accountability and efficiency.
The purchase process consists of several steps:
1. The department admin initiates the process by sending a purchase request to the IT department.
2. The IT Manager reviews the request and decides whether to approve or reject it.
3. If the request is approved, the IT Manager contacts various vendors, such as Apple, Dell, HP, ASUS, and Lenovo, to request quotes.
4. The IT Manager receives the quotes and selects the best price.
5. Once the best price is selected, the IT Manager informs the admin and waits for their approval.
6. If the admin approves, the IT Manager finalizes the vendor selection and prepares the purchase request.
7. The IT Manager then sends the purchase request to the Procurement Supervisor.
8. The Procurement Supervisor receives the request and issues a purchase order to the chosen vendor.
9. The Procurement Supervisor reviews the vendor's invoice and processing time.
10. If the processing time elapses and the tracking number has not been received, the Procurement Supervisor cancels the order.
11. If the vendor provides the tracking number within the processing time, the Procurement Supervisor collects the product once it is delivered.
12. At the same time, the Procurement Supervisor submits the payment to the vendor.
13. Once both steps are completed, the purchase process is considered finished, and the purchase is completed.
This process ensures that there is a clear and systematic approach to purchasing items, from the initial request to the final delivery and payment. Each step is important in maintaining accountability and efficiency in the procurement process.
Learn more about accountability here :-
https://brainly.com/question/29108212
#SPJ11
NEED HELP HURRY!
Select the correct answer.
Which item is made from a synthetic material?
OA
cotton pants
B.
leather jacket
O C.
tissue paper
D.
wicker basket
Answers
Answer:
B: Leather Jacket
Explanation: I hope that this helped :)
Use this graphic to explain how matter is conserved in a nuclear reaction

Answers
Nuclear processes alter the sorts of atoms present, but chemical reactions do not. The electrons in the atom play a significant role in chemical reactions when nucleus reactions occur in atom nuclei.
What is nuclear explain?The energy found in an atom's nucleus, or core, is known as nuclear energy. Energy maintains the nucleus of atoms together, the minuscule units that make up all matter in the universe. The dense nucleus of an atom has an enormous quantity of energy.
Why is a nuclear threat a threat?The most lethal weapons on earth are nuclear weapons. One can wipe out an entire metropolis, perhaps killing millions of people, endangering the ecosystem and the lives of future generations due to its long-term devastating impacts.
To know more about Nuclear visit:
https://brainly.com/question/18187269
#SPJ1
If the volume of the original sample in Part A ( P1 = 242 torr , V1 = 27.0 L ) changes to 80.0 L , without a change in the temperature or moles of gas molecules, what is the new pressure, P2 ? Express your answer with the appropriate units.
Answers
The new pressure is 81.675 torr
Since temperature and moles are held constant, we use Boyle's Law:
A gas law known as Boyle's law asserts that a gas's pressure is inversely proportional to its volume when it is held at a fixed temperature and of a given mass.
To put it another way, as long as the temperature and volume of the gas remain constant, the pressure and volume of the gas are inversely proportional to one another.
The Anglo-Irish chemist Robert Boyle proposed Boyle's law in the year 1662.
P1V1=P2V2. Simply plug in your values. The units can remain in torr. Converting to atmospheres is not needed.
(242 torr)(27.0 L)=P2(80.0 L)
P2=[(242)(27)]/80 = 81.675 torr
Hence The new pressure is 81.675 torr
Learn more about Boyle's Law here
https://brainly.com/question/26040104
#SPJ4
Write a scientific explanation that compares the electrical forces of ethanol, hexane, and water.
Claim:
Evidence:
Reasoning:
Answers
Answer:
"Water and ethyl alcohol will both have dipole-dipole interactions.
Technically they will both have Hydrogen bonding, which is a type of dipole-dipole. This is due to the high electronegativity values of oxygen atoms compared to the carbon and hydrogen atoms the oxygens bond to. This causes regions of both of these molecules that have partial negative charges and other regions wind up with partial positive charges.
Hexane will not have any dipole-dipole interactions because it is a non-polar molecule. The intermolecular forces between hexane molecules will be dispersion forces."
Explanation:
Here is the place I found the answer: https://socratic.org/questions/which-of-these-structures-has-dipole-dipole-interactions-water-h2o-ethyl-alcohol
All of this answer belongs to that person. I do not own any of this information.
The volume of a gas is 0.9 L at 273 K and 1.2 atm. What pressure will the gas occupy if the temperature is raised to 325 K and the volume is raised to 1.8 L?
Answers
when the temperature is raised to 325 K and the volume is increased to 1.8 L, the gas will occupy a pressure of approximately 1.08 atm.
To solve this problem, we can use the combined gas law equation, which relates the initial and final conditions of a gas when the amount of gas remains constant.
The combined gas law equation is given as:
(P₁ * V₁) / T₁ = (P₂ * V₂) / T₂
where P₁, V₁, and T₁ represent the initial pressure, volume, and temperature, respectively, and P₂, V₂, and T₂ represent the final pressure, volume, and temperature, respectively.
Given
Initial pressure (P₁) = 1.2 atm
Initial volume (V₁) = 0.9 L
Initial temperature (T₁) = 273 K
Final volume (V₂) = 1.8 L
Final temperature (T₂) = 325 K
We need to find the final pressure (P₂).
Substituting the given values into the combined gas law equation, we have:
(1.2 atm * 0.9 L) / 273 K = (P₂ * 1.8 L) / 325 K
Now, we can solve for P₂:
P₂ = (1.2 atm * 0.9 L * 325 K) / (273 K * 1.8 L)
= 1.08 atm
for more questions on temperature
https://brainly.com/question/2339046
#SPJ8
The outer shell of this element was determined as illustrated here. It must be a _____.
: light metal
: heavy metal
: non metal
: noble gas

Answers
The outer shell of the given element was determined as illustrated must be a noble gas. Therefore, option D is correct.
What are noble gases?Noble gases can be described as the 6 elements that are organized in group 18 of the modern periodic table. The noble gases are Helium, Neon, Argon, Krypton, Xenon, and Radon.
Under standard temperature and pressure, noble gases exist in the gaseous form and possess low reactivity. Therefore, noble gases are also referred to as inert gas. All the noble gases exhibit stable electronic configurations in their valence shell that are completely filled. Noble gases generally exist as monoatomic.
The general outer configuration of the noble gases can be written as ns²np⁶, n is the principal quantum number. Therefore, the given electron dot diagram has eight electrons in its valence shell so it is representing a noble gas.
Learn more about noble gases, here:
brainly.com/question/11764545
#SPJ2
What would the mass be, in grams, of 0.89 moles of Cl2?
Answers
Answer:
Mass = 63.19 g
Explanation:
Given data:
Number of moles of Cl₂ = 0.89 mol
Mass in gram = ?
Solution:
Formula:
Mass = number of moles × molar mass
Molar mass of Cl₂ = 71 g/mol
by putting values,
Mass = 0.89 mol × 71 g/mol
Mass = 63.19 g