Does the half life of oxygen-18 impacts its use ( explain)
Answers
Answer:
O-18 is used as a tracer in many hydrologic studies. It is most often used as a component in a mixing-model and hydrograph separation, as O-18 acts conservatively and is applied naturally and uniformly over broad areas. O-18 can be used when different sources (old water/new water) have different isotopic values.
Related Questions
compounds a, b and c each have the formula c6h12. a, b and c each react with one molar equivalent of hydrogen in the presence of a palladium catalyst to form hexane. a reacts with hbr to form a single product d. c reacts with hbr to form a single product e. b reacts with hbr to form a mixture of d and e. what is the structure of b
Answers
Structure B is a terminal alkene.
Compounds A, B, and C have the same molecular formula C6H12, So, three compounds have one double bond in their structure,
Compounds A, B, and C reacts with one molar H2 Pd to form hexane. This means all three compounds are straight chain alkenes (i.e. no branching).
Compound-A reacts with HBr to form sigle product-D, It means compound-A must be terminal alkene.
A catalyst is a substance that accelerates a chemical response, or lowers the temperature or pressure needed to begin one, with out itself being consumed in the course of the reaction.
Catalysts speed up a chemical reaction by means of reducing the amount of power you need to get one going. Catalysis is the backbone of many business tactics, which use chemical reactions to turn raw materials into beneficial products.
Catalysts may be labeled as homogeneous, heterogeneous, or enzymatic. Homogeneous catalysts exist within the identical phase because the reactants, whereas heterogeneous catalysts exist in a specific phase than the reactants.
Learn more about Catalyst here:-https://brainly.com/question/318426
#SPJ4
What is the frequency of a photon with an energy of 4.56 × 10^-19 J?OA. 6.88 x 10^14 HzOB. 6.42 x 10^14 HzOC. 4.36 x 10^14 HzOD. 5.10 x 10^14 Hz
Answers
So,
There's an equation that we could use in order to find frequency, and it is the next one:
This equation tells us that the energy of the photon is equal to the product of the Plank constant (h), which is 6.626*10^-34 J.s, and the frequency.
In this problem, we know the value of E and the value of h, so we need to solve for v:
Therefore, the correct answer option is A.


Identify the heterogeneous mixtures.
Group of answer choices
salad
water
vegetable soup
apple juice
trail mix
brass
Answers
so heterogeneous mixture is would be salad, vegetable soup, trail mix and brass.
this is because salad, vegetable soup, trail mix, and brass have a lot components and they’re not pure.
Hopefully i could help!!
what is the difference b/w combustion and synthesis reaction ?explain with example
Answers
Answer:
A synthesis reaction occurs when two or more reactants combine to form a single product in a double replacement reaction two compounds exchange elements. Combustion reaction occurs when a substance reacts quickly with oxygen combustion is commonly called burning. Example'- for synthesis reaction is iron+ oxygen —≥ water . Example:- for combustion reaction is often gaseous products.
may be this was ur answer I hope it might help u
When a person looks at a bright light, tiny muscles in the eye contract so less light can enter the eye.
Which are most likely the characteristics of this muscle? Select three options.
Answers
The three most likely characteristics of the muscle involved in controlling the amount of light entering the eye are:
NostriatedInvoluntaryAttached to eye ball. The characteristics of muscle controlling amount of light entering the eyeNonstriated: Because smooth muscles are nonstriated, they are involved in controlling how much light enters the eye. In the iris of the eye, smooth muscles are present.
Involuntary: It is the spontaneous control over the muscle contraction brought on by a bright light.
Attached to eye: ball The iris sphincter muscle is a part of the eyeball that regulates the size of the pupil, which is an opening in the iris. It surrounds the pupil and is joined to the iris, enabling it to shrink the pupil's size in reaction to light.
Learn more about muscles of the eye here
https://brainly.com/question/30558579
#SPJ1
NB: The full question
When a person looks at a bright light, tiny muscles in the eye contract so less light can enter the eye.
Which are most likely the characteristics of this muscle? Select three options.
nonstriated
involuntary
voluntary
striated
attached to skull
attached to the eyeball
Luisa has two older brothers and a younger sister. She had been learning about how to describe the ages of rocks. After thinking about it, she realized she could compare people's positions in her family in the same way that scientists compare rocks. Which geology term would describe the way Luisa compares the positions of people in her family?
a. absolute age
b. rock layers
c. relative age
d. superposition
Answers
Answer:
c. relative age
Explanation:
Relative age is a method of describing the age of rocks based on their order of appearance in the geologic column.
Events are denoted by their position in the column.
The premise of most of the assumptions used relative age determination is based on stratigraphic laws. Absolute age gives numeric ages to rocks.Since Luisa is comparing the age of people based on their position in her family, she is using the concept of relative dating.
The neutralization reaction is
H3PO4(aq) + 3 NaOH(aq) + 3H2O(l) + Na3PO4(aq)
What is the concentration of the unknown H3PO4 solution?
Answers
The concentration of the unknown \(H_{3} PO _{4}\) solution in the neutralization reaction is 4.11 × \(10^{-2}\) mol\(L^{- 1}\).
We are given the following neutralization reaction-
\(H_{3} PO _{4}\)( aq ) + 2 NaOH ( aq ) → \(Na_{2}HPO_{4}\) ( aq ) + 2 \(H_{2}O\)
Phosphoric acid is a diacid in an aqueous solution.
We have to find the concentration of the unknown \(H_{3} PO _{4}\) solution in the given neutralization reaction.
Hence, the number of moles of NaOH = 26.28 × \(10^{-3}\) L × 0.100 mol \(L^{- 1}\)
= 2.63 × \(10^{-3}\) mol
Thus, we have
the concentration \(H_{3} PO _{4}\) = 2.63 × \(10^{-3}\) mol × (1 /2) / 32.00 × \(10^{-3}\) L
= 4.11 × \(10^{-2}\) mol\(L^{- 1}\).
Hence, the concentration of the unknown \(H_{3} PO _{4}\) solution in the neutralization reaction is 4.11 × \(10^{-2}\) mol\(L^{- 1}\).
Read more about neutralization reaction:
brainly.com/question/27745033
#SPJ4
Where does the second stage of cellular respiration
occur?
W
Х
Y
Z
Please hurry this is a test

Answers
Answer:
X is your answer Have a good day
Explanation:
The pyruvate molecules from glycolysis next enter the matrix of a mitochondrion. That's where the second stage of cellular respiration takes place. This stage is called the Krebs cycle. During this stage, two more molecules of ATP are produced
Answer:
z
Explanation:
A ball is thrown straight up into the air with a speed of 13 m/s. If the ball has
a mass of 0.25 kg, how high does the ball go? Acceleration due to gravity is g = 9.8 m/s2
A. 9.2 m
B. 8.6 m
C. 10.4 m
D. 9.9 m
Answers
Answer:
The total energy, i.e. sum of kinetic and potential energy, is constant.
i.e. E = KE + PE
Initially, PE = 0 and KE = 1/2 mv^2
At maximum height, velocity=0, thus, KE = 0 and PE = mgh
Since, total energy is constant (KE converts to PE when the ball is rising),
therefore, KE = PE
or, 1/2 mv^2 = mgh
or, h = v^2 /2g = 13^2 / (2x9.8) = 8.622 m
Hope this helps.
What is your worst fear?????????????????????????????????????
Answers
Answer:
Everyone has a fear.
Fear is something that everyone have and fear itself is worse.... so there wont be any other wort fear when it itself is the worst
Answer:
apocaplyse
Explanation:
Which major branch of chemistry would be most concerned
with studying a series of chemical reactions in order to
measure the amount of heat being released in each one?
Answers
Physical chemistry a major branch of chemistry would be most concerned with studying a series of chemical reactions in order to measure the amount of heat being released in each one.
Understanding the physical characteristics of atoms and molecules, how chemical processes take place, and what these characteristics indicate are the main goals of physical chemists. Their findings are based on an understanding of chemical characteristics and a description of how they behave utilizing physics theories and mathematical calculations.Thermochemistry, which encompasses the study of the heat energy of chemical processes occurring during phase transitions like gas to liquid or vice versa, is one of the main examples of physical chemistry. It provides information on entropy, heat capacity, Gibbs free energy, or formation heat.For more information on chemistry kindly visit to
https://brainly.com/question/13428382
#SPJ1
A sample of nitrogen gas contains 8.23 x 10^23 molecules. What is the volume of this sample at STP?
Please show and explain the steps.

Answers
Answer:
30.6 L
Explanation:
Given data:
Number of molecules of nitrogen = 8.23×10²³ molecules
Volume occupy at STP = ?
Solution:
Standard temperature = 273.15 K
Standard pressure = 1 atm
One mole of any substance at STP occupy 22.4 L volume.
Number of moles of nitrogen:
1 mole contain 6.022×10²³ molecules.
8.23×10²³ molecules × 1mol / 6.022×10²³ molecules
1.37 mol
Volume of nitrogen:
1.37 mol × 22.4 L / 1 mol
30.6 L
you break down each of the following, how many differe ould you be able to recover? Mercury Sodium chloride
Answers
Answer:
When the term "break down" is used in reference to substances, it typically means to chemically decompose or separate a compound into its individual elements or molecules. In the case of Mercury and Sodium chloride, the number of different substances that can be recovered from the breakdown would depend on the method of breaking down or separation used.
Mercury is a chemical element with the atomic number 80, and it is typically found as a liquid at standard conditions for temperature and pressure. Mercury can be broken down into its individual atoms through a process called electrolysis, which uses an electrical current to split the mercury atoms into their component elements. Therefore, if mercury were broken down through electrolysis, the only substance that could be recovered would be individual mercury atoms.
Sodium chloride, on the other hand, is an ionic compound consisting of sodium cations (Na+) and chloride anions (Cl-). It is commonly known as table salt and is a crystalline solid at room temperature. Sodium chloride can be broken down into its individual ions through a process called electrolysis, similar to the breakdown of mercury. Therefore, if sodium chloride were broken down through electrolysis, two different substances could be recovered: sodium ions and chloride ions.
In addition, sodium chloride can also be broken down into its individual elements using a more traditional chemical reaction known as a decomposition reaction. This involves heating sodium chloride to high temperatures to break the ionic bond between sodium and chlorine. In this case, two different substances could also be recovered: metallic sodium and chlorine gas.
In summary, the number of different substances that could be recovered from the breakdown of Mercury and Sodium chloride would depend on the method of breaking down or separation used. In the case of electrolysis, only individual atoms or ions could be recovered, while in the case of decomposition or heating, unique elements or gases could be retrieved.
Q-3 Determine the fugacity in atm for pure ethane at 310 K and 20.4 atm and change in the chemical potential between this state and a second state od ethane where temperature is constant but pressure is 24 atm.
Answers
The fugacity in atm for pure ethane at 310 K and 20.4 atm is given by the equation: f = 20.4 exp (-Δg1/RT). The change in chemical potential between this state and a second state of ethane where the temperature is constant but the pressure is 24 atm is -0.0911RT.
Fugacity is a measure of the escaping tendency of a component in a mixture, which is defined as the pressure that the component would have if it obeyed ideal gas laws. It is used as a correction factor in the calculation of equilibrium constants and thermodynamic properties such as chemical potential. Here we need to determine the fugacity in atm for pure ethane at 310 K and 20.4 atm and the change in the chemical potential between this state and a second state of ethane where the temperature is constant but the pressure is 24 atm. So, using the formula of fugacity: f = P.exp(Δu/RT) Where P is the pressure of the system, R is the gas constant, T is the temperature of the system, Δu is the change in chemical potential of the system. Δu = RT ln (f / P)The chemical potential at the initial state can be calculated using the ideal gas equation as: PV = nRT
=> P
= nRT/V
=> 20.4 atm
= nRT/V
=> n/V
= 20.4/RT The chemical potential of the system at the initial state is:
Δu1 = RT ln (f/P)
= RT ln (f/20.4) Also, we know that for a pure substance,
Δu = Δg. So,
Δg1 = Δu1 The change in pressure is 24 atm – 20.4 atm
= 3.6 atm At the second state, the pressure is 24 atm.
Using the ideal gas equation, n/V = 24/RT The chemical potential of the system at the second state is: Δu2 = RT ln (f/24) = RT ln (f/24) The change in chemical potential is Δu2 – Δu1 The change in chemical potential is
Δu2 – Δu1 = RT ln (f/24) – RT ln (f/20.4)
= RT ln [(f/24)/(f/20.4)]
= RT ln (20.4/24)
= - 0.0911 RT Therefore, the fugacity in atm for pure ethane at 310 K and 20.4 atm is:
f = P.exp(Δu/RT)
=> f
= 20.4 exp (-Δu1/RT)
=> f
= 20.4 exp (-Δg1/RT) And, the change in the chemical potential between this state and a second state of ethane where the temperature is constant but pressure is 24 atm is -0.0911RT. Therefore, the fugacity in atm for pure ethane at 310 K and 20.4 atm is given by the equation: f = 20.4 exp (-Δg1/RT). The change in chemical potential between this state and a second state of ethane where the temperature is constant but the pressure is 24 atm is -0.0911RT.
To know more about chemical potential visit:-
https://brainly.com/question/31100203
#SPJ11
which of the following acids is strongest, based on the values of their acid ionization constants? benzoic acid carbonic acid sulfuric acid hydrazoic acid oxalic acid
Answers
The strongest acid among the following is sulfuric acid, based on the values of their acid ionization constants. Sulfuric acid is a diprotic acid that has two acidic hydrogen atoms, so it has two ionization constants.What is an acid ionization constant
An acid ionization constant (Ka) is a quantitative measure of the strength of an acid in a solution. A high Ka value indicates that an acid will completely ionize in a solution, whereas a low Ka value indicates that an acid will partially ionize in a solution.How can we compare the strength of different acids based on their ionization constants?The ionization constants of different acids can be compared to determine their relative strength. The higher the ionization constant, the stronger the acid. For example, if acid A has an ionization constant of 1 x 10-4 and acid B has an ionization constant of 1 x 10-6, acid A is stronger because it has a higher ionization constant.Now, let's look at the given options and their acid ionization constants:Benzoic acid: Ka = 6.4 × 10-5Carbonic acid: Ka1 = 4.2 × 10-7 and Ka2 = 4.8 × 10-11Hydrazoic acid: Ka = 1.9 × 10-5Oxalic acid: Ka1 = 5.9 × 10-2 and Ka2 = 6.4 × 10-5Sulfuric acid: Ka1 = 1.0 × 103 and Ka2 = 1.2 × 10-2Therefore, we can see that the ionization constant of sulfuric acid is the strongest, based on the values of their acid ionization constants.
To know more about chemical equation, visit ;
https://brainly.com/question/11231920
#SPJ11
karl-anthony is trying to plate gold onto his silver ring. he constructs an electrolytic cell using his ring as one of the electrodes. he runs this cell for 94.7 minutes at 220.8 ma. how many moles of electrons were transferred in this process?
Answers
0.11 moles of electrons were transferred during the electroplating process.
The number of moles of electrons transferred can be calculated using Faraday's constant, which represents the amount of charge carried by one mole of electrons.
Faraday's constant is approximately 96,485 C/mol. Using this constant and the given information, the number of moles of electrons transferred can be calculated as:
moles of electrons = (220.8 mA * 94.7 min * 60 s/min) / (1000 mA/A * 96,485 C/mol)moles of electrons = 0.11 molTherefore, 0.11 moles of electrons were transferred during the electroplating process.
To learn more about Faraday's constant, here
https://brainly.com/question/29290837
#SPJ4
what are 5 physical changes in matter
Answers
Answer:
cutting, bending, dissolving, freezing, and boiling
Explanation:
A physical change is a change in one or more physical properties of matter without any change in chemical properties. In other words, matter doesn't change into a different substance in a physical change. Examples of physical change include but are not limited to, from solid to liquid or from liquid to gas are also physical changes.
ANYONE PLEASE HELP ME IN CHEMISTRY I REALLY NEED THE ANSWER RIGHT NOW BECAUSE I HAVE TO PASS THIS TOMORROW AT 4TH PERIOD I HOPE Y'ALL CAN HELP ME:(
I'LL MARK YOU AS THE BRAINLIEST!

Answers
Answer:
1)30
2)
3)32.3
4)20.8
5)52.5
6)42.6
7)
8)11
9)3.5
10)12.75
Explanation:
please give me brainlest and follow me
the blank spaces are which I don't understand that diagram
According to the spectrum shift in this image, the star we are looking at is __________________________ and moving ______________________ us.
options:
blue-shifting ; closer to
blue-shifting ; further from
red-shifting ; closer to
red-shifting ; further from
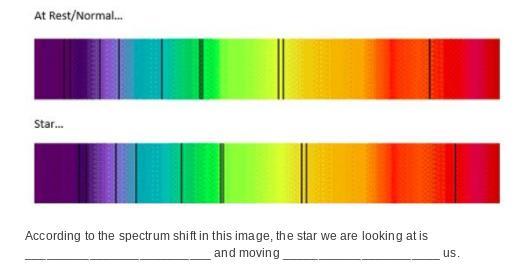
Answers
Answer: D
Explanation:
The lab frame is the same as the rest frame. In the lab, we see the first spectrum. But when we look at the star, we record the second spectrum. When stars move away from us, the light that they emit is red shifted. You can see this by comparing the lines, which are shifting towards the red part of the spectrum.
arrange the following gases in order of increasing rate of effusion: c2h6, ar, hcl, ph3
Answers
The order of increasing rate of effusion is: C₂H₆ < PH₃ < HCl < Ar.
The rate of effusion for a gas depends on its molar mass and the temperature. According to Graham's law of effusion, the rate of effusion is inversely proportional to the square root of the molar mass. Therefore, gases with lower molar masses will effuse faster than those with higher molar masses at the same temperature.
To arrange the gases in order of increasing rate of effusion, we need to compare their molar masses:
Ar (argon): Molar mass = 39.95 g/mol
HCl (hydrogen chloride): Molar mass = 36.46 g/mol
PH₃ (phosphine): Molar mass = 33.99 g/mol
C₂H₆ (ethane): Molar mass = 30.07 g/mol
Now, we can compare the molar masses and determine the order of increasing rate of effusion:
C₂H₆ (ethane): It has the lowest molar mass among the given gases, so it will have the highest rate of effusion.
PH₃ (phosphine): It has a higher molar mass than ethane but lower than hydrogen chloride and argon. Therefore, it will have a higher rate of effusion compared to hydrogen chloride and argon but lower than ethane.
HCl (hydrogen chloride): It has a higher molar mass than both ethane and phosphine. Hence, it will have a lower rate of effusion than ethane and phosphine.
Ar (argon): It has the highest molar mass among the given gases, so it will have the lowest rate of effusion.
Therefore, the order of increasing rate of effusion is:
C₂H₆ < PH₃ < HCl < Ar
In summary, ethane (C₂H₆) will have the highest rate of effusion, followed by phosphine (PH₃), hydrogen chloride (HCl), and finally, argon (Ar), which will have the lowest rate of effusion.
To know more about Graham's law of effusion, refer to the link below:
https://brainly.com/question/19589867#
#SPJ11
Mercury poisoning is a debilitating disease that is often fatal. In the human body, mercury reacts with essential enzymes leading to irreversible inactivity of these enzymes. If the amount of mercury in a polluted lake is 0.4 Hg/mL, what is the total mass in kilograms of mercury in the lake
Answers
Answer:
The total mass of mercury in the lake is 631,542.7 kg
Explanation:
Question: The given dimensions of the lake as obtained from a similar question posted online are;
The surface area of the lake, A = 100 mi²
The lake's average depth, d = 20 ft.
The concentration of the mercury, C = 0.4 μg Hg/mL = 0.4 × 10⁻⁶ kg Hg/L
Therefore, we have;
The volume of water mixture in the lake, V = A × d
∴ V = 100 mi² × 20 ft. = 2,787,840,000 ft.² × 20 ft. = 55,756,800,000 ft.³
1 ft³ = 28.31685 L
∴ 55,756,800,000 ft.³ = 55,756,800,000 ft.³ × 28.31685 L/ft.³ = 1.57885675 × 10¹² L
The total mass of mercury in the lake, m = C × V
∴ m = 0.4 × 10⁻⁶ kg Hg/L × 1.57885675 × 10¹² L = 631,542.7 kg
The total mass of mercury in the lake, m = 631,542.7 kg.
Which set of numbers will balance the following equations? 1's have been included for clarity.__Mn3N4 + __NaF --> __MnF4 + __Na3N a 1; 4; 1; 4 b 1; 4; 3; 2 c 1; 12; 3; 4 d 3; 2; 3; 2
Answers
ANSWER
Option C
EXPLANATION
Given that;
\(\text{ ----- Mn}_3N_4\text{ }+\text{ ---- NaF }\rightarrow\text{ ---- MnF}_4\text{ }+\text{ ---Na}_3N\)In the reaction above, we have the following data
At the reactants side;
3 atoms of manganese
4 atoms of nitrogen
1 atom of sodium
1 atom of fluorine
At the products side
1 atom of manganese
4 atoms of fluorine
3 atoms of sodium
1 atom of nitrogen
To balance the above equation, apply the law of conservation mass
Law of conservation of mass states that matter can neither be created nor destroyed but can e transformed from one formato another.
To balance the equation, 1 mole of Mn3N4 reacts with 12 moles of Na Tto give 3 moles of MnF4 and 4 moles of Na3N
So, the new equation becomes
\(\text{ Mn}_3N_4\text{ }+\text{ 12NaF }\rightarrow\text{ 3MnF}_4\text{ }+\text{ 4Na}_3N\)The following data can be deduced in the above equation
At the reactants side
3 atoms of Mn
4 atoms of N
12 atoms of Na
12 atoms of F
At the products side
3 atoms of Mn
12 atoms of F
12 atoms of Na
4 atoms of N
Looking atthe vabove data, the number of atoms of each element at the reactants side is equal to the number of atoms of same elements at the products side.
Hence, the correct answer is option Ce
u
Reactions review help please

Answers
The answer response are:
The mass of the reactants must equal the mass of the products in a chemical reaction. A chemical reaction is indicated by a color change, bubbles, or a change in temperature. Mass is the measure of the amount of matter in a substance. A chemical equation must have the same number of reactants and products to be balanced. An element is a pure substance made up of only one type of atom. A molecule is the smallest part of a compound that retains the chemical properties of that compound. An example of a chemical equation is CH₂ + 2 O₂ → CO₂ + 2H₂O. Melting point, phase change, magnetism, and buoyancy are all examples of physical properties of matter. NaCl (sodium chloride) and FeSO (iron(II) sulfate) are examples of compounds. The "2" in H₂O and the "3" in CaCO₃ are examples of subscripts, which indicate the number of atoms in a molecule.What is thechemical reactions about?Chemical reactions are the processes by which atoms or groups of atoms are rearranged to form new substances with different properties. During a chemical reaction, the bonds between atoms are broken and new bonds are formed.
The reactants are the starting materials, and the products are the new substances that are formed. Chemical reactions are essential to many natural and industrial processes, including photosynthesis, combustion, corrosion, and the production of chemicals and pharmaceuticals.
Therefore, Understanding chemical reactions helps us explain the complexity of our world and develop new technologies to solve problems and improve our lives.
Learn more about chemical equation from
https://brainly.com/question/11231920
#SPJ1
In the product 6O2, what does the coefficient mean?
Answers:
There are 12 molecules of O2,
There are 8 molecules of O2,
There are 6 molecules of O2.
There are 2 molecules of O2.
Answers
Answer:
(A) there are 12
Explanation:
bc
What type of bond results from the side -on overlap of orbitals?
O a (sigma) bond
O ionic bond
O r (pi) bond
O hydrogen bond
Answers
The type of bond that results from the side-on overlap of orbitals is a pi (π) bond.
In chemical bonding, the side-on overlap of orbitals occurs when parallel p orbitals align and share electron density. This type of overlap is characteristic of pi (π) bonding.
Pi (π) bonds are formed in addition to sigma (σ) bonds, which result from the head-on overlap of orbitals. Unlike sigma bonds that allow rotation, pi bonds are formed by the sideways overlap of p orbitals and restrict rotation around the bond axis.
Pi bonds are commonly observed in molecules with double or triple bonds, such as alkenes and alkynes. The additional overlap of p orbitals in these molecules creates the pi-bonding framework, which adds strength and stability to the overall molecular structure.
It is important to note that ionic bonds involve the complete transfer of electrons between atoms, while hydrogen bonds are weaker electrostatic attractions between a hydrogen atom and an electronegative atom. Neither of these bond types are directly associated with the side-on overlap of orbitals.
learn more about pi (π) bond here:
https://brainly.com/question/32547611
#SPJ11
I WILL MARK BRAINLIEST!!!!! !!
(picture included with one question ^^^^^^^)

Answers
Answer:
type D
balance form .b
Explanation:
In the current periodic table, how are the elements arranged?
a. the bonding power with oxygen
b. the number of neutrons
c. atomic mass
d. atomic number
Answers
Answer:
The answer would be
C. The number of neutrons
You're welcome <3
PLEASE ANSWER!!!! 30 POINTS!!!!!!!
How many grams of NH3 form when 84 g N2 react completely?
3H2 + N2 ---> 2NH3
N2: 28 g/mol NH3: 17 g/mol
84 g N2 ---> g NH3
Answers
Answer: 84 g of N2 reacts completely to form 102 g of NH3.
Explanation: Change over the mass of N2 from grams to moles utilizing its molar mass:
84 g N2 × (1 mol N2 / 28 g N2) = 3 moles of N2
Utilize stoichiometry to calculate the number of moles of NH3 delivered, knowing that 3 moles of H2 are required to respond with 1 mole of N2:
3 moles of N2 × (2 moles of NH3 / 1 mole of N2) = 6 moles of NH3
Change over the number of moles of NH3 to grams utilizing its molar mass:
6 moles of NH3 × (17 g NH3 / 1 mol NH3) = 102 g NH3
Can you guys please help me with this chart PLEASEEE?

Answers

for the following word equation, write it as a chemical equation, then balance it
potassium + oxygen gas----- potassium oxide
Answers
Answer:
4K + O2 ---> 2K2O
Explanation:
The valence of Potassium is +1
The valence of oxygen is -2
Thus, two atoms of potassium will attach with one atom of oxygen to form molecule of potassium oxide.
This can be written as
2K + O ---> K2O
However, this is not the balanced equation as oxygen exists as O2 molecule.
Thus, the balanced equation is
4K + O2 ---> 2K2O