A sample of O2 under 2.31 atm occupies 525 ml at 25.0°C. What volume will it occupy at 10.5 atm at the same temperature?
Answers
At the same temperature, the O2 sample will occupy a volume of approximately 121.9 mL at 10.5 atm.
To solve this problem, you can use the Gas Law formula: P1V1/T1 = P2V2/T2. In this case, the temperature remains constant, so we can simplify the equation to P1V1 = P2V2. Where P1, V1, and T1 are the initial pressure, volume, and temperature, and P2 and V2 are the final pressure and volume
Given:
P1 = 2.31 atm
V1 = 525 mL
P2 = 10.5 atm
Now, we need to find V2:
2.31 atm * 525 mL = 10.5 atm * V2
Solving for V2:
V2 = (2.31 * 525) / 10.5
V2 ≈ 121.9 mL
So, the O2 sample will occupy approximately 121.9 mL at 10.5 atm at the same temperature.
More on gas volume: https://brainly.com/question/23007892
#SPJ11
Related Questions
any ski resorts and mountain cities can be reached directly by planes which deposit travelers a mile or more above sea level. This can result in altitude sickness due to hypoxemia, or reduced oxygen in the blood, caused by the unaccustomed exposure to the lower atmospheric pressure at high elevations.
On a given day, the prevailing atmospheric pressure in Mexico City (elevation 2240 meters) might be 0.754 atm. Calculate this pressure expressed in mmHg and in torr.
Answers
It's important for travelers to acclimatize themselves gradually to high altitudes, stay hydrated, and avoid alcohol and strenuous physical activity until their body adjusts to the lower oxygen levels. If symptoms persist or worsen, medical attention should be sought immediately.
The prevailing atmospheric pressure in Mexico City from atm to mmHg and torr,
1 atm = 760 mmHg
1 atm = 760 torr
Therefore, to convert 0.754 atm to mmHg, we can multiply it by the conversion factor:
0.754 atm x 760 mmHg/atm = 573.44 mmHg
So the prevailing atmospheric pressure in Mexico City is 573.44 mmHg.
0.754 atm x 760 torr/atm = 573.44 torr
So the prevailing atmospheric pressure in Mexico City is 573.44 torr.
To know more about oxygen visit :-
https://brainly.com/question/14713109
#SPJ11
please help me
16 1 point What is the decay rate of a sample of Oxygen-21 if the sample has 8.31x1017 atoms and a decay constant of 0.203/s? 4.09x1018Bq 1.69x10¹7Bq 0.203Bq 2.44x10-1⁹Bq Previous
Answers
decay rate of approximately 1.69x10^17 Bq (becquerels),
The decay rate of a radioactive sample is determined by the number of radioactive atoms present and the decay constant, which represents the probability of decay per unit of time.
To calculate the decay rate, we multiply the number of atoms in the sample by the decay constant. In this case, the sample has 8.31x10^17 atoms and a decay constant of 0.203/s. Multiplying these values gives a decay rate of approximately 1.69x10^17 Bq (becquerels), which represents the number of decays per second in the sample.
Learn more about Oxygen here : brainly.com/question/13905823
#SPJ11
Round to 3 significant figures.
1.235
Answers
Answer:
1.24
Explanation:
According to the concept of significant figures, rounding off to 3 significant figures gives 1.24.
What are significant figures?Significant figures are used for establishment of a number which is presented in the form of digits. These digits give a meaningful representation to the numbers.
The significant figures are the significant digits which convey the meaning according to the accuracy. These provide precision to the numbers and hence are called as significant numbers.There are rules for counting significant figures which are as follows:
1)All non-zero digits are significant .
2)All zeroes which occur between non-zero digits are significant.
3)All zeroes to the left and right of a non-zero digit are not significant.
4) All zeroes on right of decimal are significant if a non-zero number follows them.
5)All zeroes on right side of non-zero digit are significant.
Learn more about significant figures,here:
https://brainly.com/question/29153641
#SPJ7
What happens to the heat energy that leaves the milk?
Answers
Answer:
it gets cold or warm
Explanation:
pleas get me 53 likes
If the ksp for ca3(po4)2 is 8. 6×10−19, and the calcium ion concentration in solution is 0. 0023 m, what does the phosphate concentration need to be for a precipitate to occur?
Answers
The answer is the phosphate concentration of \(8.40\times10^{-6}M\)
Calcium phosphate \($\left(\mathrm{Ca}_{3}\left(\mathrm{PO}_{4}\right)_{2}\right)$\) is dissociate to form
calciumion \($\left(\mathrm{Ca}^{2+}\right)$\) and phosphate ion \($\left(\mathrm{PO}_{4}^{3-}\right)$\).
\($\mathrm{Ca}_{2}\left(\mathrm{PO}_{4}\right)_{2}(\mathrm{~s}) \longrightarrow 3 \mathrm{Ca}^{2+}(\mathrm{aq})+2 \mathrm{PO}_{4}^{3-}(\mathrm{aq})$\)
Soluability product expression of \($\mathrm{Ca}_{3}\left(\mathrm{PO}_{4}\right)_{2}$\) is,
\($K_{s p}=\left[\mathrm{Ca}^{2+}\right]^{3} \times\left[\mathrm{PO}_{4}^{3-}\right]^{2} \longrightarrow (1)\)
given that, somability product \($\left(k_{s p}\right)=8.6 \times 10^{-19}$\)
concentration of \(\mathrm{ca}^{2+}$ ion $=0.0023 \mathrm{M}$\)
putting the value in equation (1) we get,
\($8.6 \times 10^{-19}=(0.0023)^{3} \times\left[\mathrm{PO}_{4}^{3-}\right]^{2}$\)
or, \($\left[\mathrm{PO}_{4}^{3-}\right]^{2}=\frac{8.6 \times 10^{-19}}{(0.0023)^{3}}=7.06 \times 10^{-11}$\)
or, \($\left[\mathrm{PO}_{4}^{3-}\right]=8.40 \times 10^{-6} \mathrm{M}$\)
So the phosphate concentration \($=8.40 \times 10^{-6} \mathrm{M}$\)
What is phosphate concentration ?
Phosphorus is a component of adenosine diphosphate (ADP) and adenosine triphosphate (ATP), both of which utilise the bonds formed between phosphate groups to store energy. Although it is also found in plasma, phosphate is a significant intracellular anion. Adults' typical serum phosphate concentrations fall between\(2.5$ to $4.5 \mathrm{mg} / \mathrm{dL}(0.81$ to $1.45 \mathrm{mmol} / \mathrm{L})$\)So the more about phosphate concentration visit.
https://brainly.com/question/13606926
#SPJ4
Rank the following bonds by increasing price volatility (duration). 1) \( 2,4,3,1 \) 2) \( 4,2,1,3 \) 3) \( 3,2,4,1 \) 4) \( 4,3,1,2 \) 5) \( 2,3,4,1 \)
Answers
The ranking of bonds by increasing price volatility (duration) is as follows:
2) 4,2,1,3
This means that option 2 ranks the bonds in the correct order of increasing price volatility.
The duration of a bond measures its sensitivity to changes in interest rates. Generally, bonds with longer durations are more sensitive to interest rate changes and exhibit greater price volatility.
In the given ranking, the bond with the lowest price volatility (shortest duration) is bond 4, followed by bond 2, bond 1, and bond 3. This implies that bond 4 is the least affected by interest rate changes and has the lowest price volatility, while bond 3 is the most sensitive to interest rate changes and has the highest price volatility.
The ranking is based on the understanding that longer-term bonds tend to have higher durations and are more susceptible to price fluctuations due to changes in interest rates, while shorter-term bonds have lower durations and exhibit lower price volatility.
learn more about bonds here:
https://brainly.com/question/28299101
#SPJ11
Purifying polluted water

Answers
The most effective water filtration systems to guard against these harmful effects use natural materials like carbon, ceramic, and sand.
Never put water that is warmer than 35 °C through the filter cartridge. Under no circumstances should you run water through the appliance that is over 50 °C. Avoid keeping purified water in storage.
Through adjusting the electrostatic charges of particles suspended in water, the chemical water treatment process known as coagulation removes solids from water.
The term "floc" refers to the "snowballing" of microscopic particles into bigger ones. It is a time-dependent procedure that has a direct impact on the effectiveness of clarity by giving particles suspended in water several chances to clash through slow, sustained agitation.
A water treatment facilities employ sedimentation as one of their processes for separating particulates from water.
Filtration is the process of removing solid particles from liquid or gaseous fluids by passing the fluid through a filter medium while keeping the solid particles behind.
Learn more about water filtration, here:
https://brainly.com/question/30313108
#SPJ1
CaCOs(s) -» CaO(s) + C02(g)
(3)
A student heats a 5 g pellet of CaCO3 strongly for 10 minutes over a Bunsen burner flame.
After cooling, the student uses an open container to measure the mass of the products formed and finds this to be 2.8 g.
Provide an explanation for the decrease in mass of the products compared to the reactant and work out how many molecules of carbon dioxide would form in this reaction.
Answers
CaCO₃(s) → CaO(s) + CO₂(g) is tha balanced chemical reaction. One molecule of carbon dioxide would form in this reaction.
What is balanced chemical reaction ?A balanced chemical equation is defined as an equation where the number of atoms of type in the reaction is the equal on both reactants and product sides. The mass, as well as the change, are similar in a balanced chemical equation.
If a student heats a 5 g pellet of CaCO₃ strongly for 10 minutes complete a Bunsen burner flame. After cooling, the student utilizes an open container to decide the mass of the products formed and finds this to be 2.8 g.
The decrease in mass of the products compared to the reactant because there is formation of carbon dioxide as a side product.
Thus, There are one molecule of CO₂ is produced.
To learn more about the balanced chemical reaction, follow the link;
https://brainly.com/question/30230799
#SPJ9
Which of the following correctly compares glass A and glass B?
A black line splitting the image into two parts. On the left hand side is glass A that is almost full. On the right side is glass B that is half full.
Question 13 options:
Glass A has the same mass as glass B.
Glass A has a greater mass than glass B.
Glass A has the same amount of liquid as glass B.
The liquid in both glasses takes up the same space.

Answers
Answer:
Glass A has a greater mass than glass B
Answer:
Glass A has a greater mass than Glass B
hope this helped :>
A cereal contains 11.0 grams of sucrose (C12H22O11) per 60.0 grams of cereal. How many grams of cereal must be eaten to consume 0.0424 moles of sucrose? Please write it out to fit in the template.
Answers
The grams of cereal that must be eaten to consume 0.0424 moles of sucrose is 79.14 grams.
The number of grams of cereal that must be eaten to consume 0.0424 moles of sucrose can be calculated as follows :
Given : Sucrose = 0.0424 moles ; Molar mass of sucrose, C12H22O11 = 342 g/mol
To find the mass of sucrose :
Mass of Sucrose = molar mass × number of moles of sucrose
= 342 g/mol × 0.0424 mol = 14.51 g
Now we can calculate the grams of cereal
11 grams of sucrose are present in 60.0 grams of cereal.
14.51 grams of sucrose are present in cereal
Mass of Cereal containing 14.51 g of sucrose = 14.51 × 60 / 11 = 79.14 grams
Therefore, the grams of cereal that must be eaten to consume 0.0424 moles of sucrose is 79.14 grams.
To learn more about moles :
https://brainly.com/question/29367909
#SPJ11
Magnesium oxide (mgo) forms when the metal magnesium burns in air.(a) if 1.18 g of mgo contains 0.712 g of mg, what is the mass ratio of magnesiu?
Answers
The mass ratio of magnesium in magnesium oxide (MgO) can be calculated by dividing the mass of magnesium (0.712 g) by the mass of magnesium oxide (1.18 g).
To find the mass ratio, we divide the mass of the element of interest (magnesium) by the mass of the compound (magnesium oxide). In this case, the mass of magnesium is given as 0.712 g and the mass of magnesium oxide is given as 1.18 g. So, the mass ratio of magnesium is calculated as follows:
Mass ratio = mass of magnesium / mass of magnesium oxide
= 0.712 g / 1.18 g
Calculating this gives us the mass ratio of 0.604.
Therefore, the mass ratio of magnesium in magnesium oxide is approximately 0.604.
The mass ratio of magnesium in magnesium oxide can be found by dividing the mass of magnesium by the mass of magnesium oxide. In this case, the mass of magnesium is given as 0.712 g and the mass of magnesium oxide is given as 1.18 g. By dividing these two values, we get a mass ratio of approximately 0.604. This means that for every gram of magnesium oxide, there are approximately 0.604 grams of magnesium. This mass ratio is useful in determining the composition of compounds and can be used in various chemical calculations.
To know more about magnesium visit:
https://brainly.com/question/8351050
#SPJ11
true or false: wood-to-energy systems release carbon dioxide so it cannot be considered a renewable energy source.
Answers
False. wood to energy source can release carbon dioxide when it is burned.so it can be considered as a renewable energy source.
Burning wood can release lots of carbon dioxide comparatively to other fossil fuels. plants and trees are considered as renewable energy source. because when it is cut again it grows. its is less dense energy source. Many solar power plants can convert carbon dioxide into fuels. and it can be recycle to other chemicals. Renewable energy emits between 11 to 750 g of carbon dioxide. As long as trees planted its a great source of renewable energy source. This also have many other environmental benefits.
To learn more about Renewable energy please visit:
https://brainly.com/question/79953
#SPJ4
if a material is ohmic what do you expect the reistance to be
Answers
If a material is ohmic, the resistance is expected to be constant. An ohmic material is a material that follows Ohm's law. In other words, the electric current flowing through the material is proportional to the potential difference or voltage applied across the material at a constant temperature and pressure.
The resistance of the ohmic material is defined by the ratio of the potential difference applied to the current flowing through the material. If a material follows Ohm's law, it is considered to be an Ohmic material. In ohmic materials, the resistance is constant, meaning it doesn't vary as the voltage and current change. If the current through an ohmic material is doubled, the voltage across the material is also doubled.
Hence, the resistance of the material remains constant. In contrast, the resistance of non-ohmic materials varies with voltage and current. Resistance is the degree to which an object opposes the flow of electrical current. The resistance of a material depends on its physical properties such as length, cross-sectional area, and temperature. The resistance of a conductor is defined by the ratio of the voltage applied across it to the current that flows through it. The unit of resistance is ohms (Ω).
To know more about resistance refer to:
https://brainly.com/question/32678336
#SPJ11
Which of the following is(are) used in the overall reactions for photosynthesis?
Answers
Answer:
Photosynthesis takes the energy of sunlight and combines water and carbon dioxide to produce sugar and oxygen as a waste product. The reactions of respiration take sugar and consume oxygen to break it down into carbon dioxide and water, releasing energy.
Explanation:
a buffer is a substance that releases hydrogen ions if a solution becomes too acidic. releases hydrogen ions when base is added to a solution. converts excess hydroxide ions into hydrogen ions to maintain ph. absorbs hydrogen ions if a solution becomes too basic.
Answers
A buffer is a substance that maintains the pH of a solution by either releasing or absorbing hydrogen ions (H+) depending on the conditions.
Buffers play a crucial role in maintaining the pH of a solution, which is a measure of its acidity or basicity. They help prevent large fluctuations in pH by acting as a reservoir for hydrogen ions. In the context of the given options, a buffer performs multiple functions:
1. A buffer releases hydrogen ions if a solution becomes too acidic: When the concentration of hydrogen ions increases, indicating acidity, a buffer can release additional hydrogen ions to counterbalance the excess, preventing a drastic decrease in pH.
2. A buffer releases hydrogen ions when a base is added to a solution: When a base is added to a solution, it reacts with the hydrogen ions present. A buffer can release additional hydrogen ions to neutralize the base and maintain the pH within a certain range.
3. A buffer converts excess hydroxide ions into hydrogen ions to maintain pH: If the concentration of hydroxide ions (OH-) increases, indicating basicity, a buffer can convert the excess hydroxide ions into water by accepting hydrogen ions. This helps prevent the pH from rising too high.
Overall, buffers act as pH regulators, maintaining a relatively stable pH in a solution by either releasing or absorbing hydrogen ions depending on the circumstances. This ability to resist changes in pH is essential in biological systems and many chemical processes.
Learn more about Hydrogen
brainly.com/question/30623765
brainly.com/question/31485826
#SPJ11
Cual es la masa atómica del fósforo si tiene 16 neutrones?
Answers
Answer:
nbfuhbcuwcbevbeufvhev
Explanation:
jejfihfirhugv
If a cell inside of an organism were unable to exchange food, water, and other materials with its environment, what would happen to it? It would produce new cells. It would maintain homeostasis. It would not be able to survive. It would move to a new location.
Answers
Answer:
It would not be able to survive
Explanation:
A cell can only survive by exchanging materials with its environment. exchange of materials is necessary for life.
For instance, a cell gives out carbon dioxide and takes in oxygen which is necessary for life.
Most cells depend on exchange of materials with their environment for nutrition.
Classify each property according to whether it is displayed by metals or by nonmetals
a. low melting point b. shiny dull c. poor conductor d. ductile e. malleable f. brittle g. good conductor h. high melting point
Answers
a. low melting point: Nonmetals. b. shiny: Metals. c. poor conductor: Nonmetals. d. ductile: Metals. e. malleable: Metals. f. brittle: Nonmetals. g. good conductor: Metals. h. high melting point: Metals
a. Low melting point is generally displayed by nonmetals. Metals tend to have high melting points.
b. Metals are typically shiny due to their ability to reflect light.
c. Nonmetals are generally poor conductors of heat and electricity.
d. Metals are ductile, meaning they can be drawn into thin wires without breaking.
e. Metals are malleable, meaning they can be hammered into thin sheets or shapes without shattering.
f. Nonmetals are usually brittle, meaning they are prone to breaking or shattering when subjected to stress.
g. Metals are good conductors of heat and electricity.
h. Metals typically have high melting points compared to nonmetals.
It's important to note that these are general trends and not absolute characteristics for all metals or nonmetals. Some exceptions or variations may exist within specific elements or compounds.
Learn more about properties of metals and nonmetals here: brainly.com/question/638020
#SPJ11
Draw the expanded structural formula for the condensed formula (CH3)2CHCH2OCH2CH3 . Draw all hydrogen atoms
Answers
We have that the Complete Expanded Structure of (CH3)2CHCH2OCH2CH3 is given in the attachment below
From the Question
(CH3)2CHCH2OCH2CH3
Generally for the condensed formula (CH3)2CHCH2OCH2CH3
We consider that this is a single bond connecting them
We consider
Hydrogen H(1)
Oxygen(8)
Carbon(6)
In conclusion
The Complete Expanded Structure of (CH3)2CHCH2OCH2CH3 is given in the attachment below.
For more information on this visit
https://brainly.com/question/24102840

Which one of the following statements about amorphous ceramics and crystalline ceramics is not correct? .Amorphous ceramics has low melting temperature, while crystalline ceramics has high melting temperature .Processing crystalline ceramics is very similar to processing powder metal components .Amorphous Ceramics have higher melting temperature, while crystalline ceramics have lower melting temperature
Answers
The statement that is not correct about amorphous ceramics and crystalline ceramics is: "Amorphous Ceramics have higher melting temperature, while crystalline ceramics have lower melting temperature."
Amorphous ceramics typically have lower melting temperatures compared to crystalline ceramics because of their disordered atomic structure, which requires less energy to break their bonds. Crystalline ceramics, on the other hand, have a well-ordered atomic structure, resulting in higher melting temperatures due to the need for more energy to break the stronger bonds.
The incorrect statement is the one suggesting that amorphous ceramics have a higher melting temperature than crystalline ceramics. In reality, amorphous ceramics generally have a lower melting temperature than their crystalline counterparts.
To know more about Amorphous ceramics, click here
https://brainly.com/question/24182464
#SPJ11
The reaction A( g ) ⇌ 2 B( g ) A(g) ⇌ 2 B(g) has an equilibrium constant of K = 0.010 K = 0.010. What is the equilibrium constant for the reaction B( g ) ⇌ 1 2 A( g ) ?
Answers
Answer:
K = 10
Explanation:
Using Hess's law, it is possible to obtain the equilibrium constant, K, of a reaction using K of similar reactions. For example:
If A ⇄ B K = X
B ⇄ A K = 1/X
2A ⇄ 2B K = X².
Thus, if A(g) ⇄ 2B(g) K = 0.010
2B(g) ⇄ A(g) K = 1 / 0.010; K = 100
B(g) ⇄ A(g) K = √100 = 10
K = 10select all the approximate bond angles between bonding domains that appear in the following molecular geometry
Answers
90° ,109.5°, 120°, 180° are the appropriate bond angles between bonding domains that appear in the following molecular geometry.
In molecular geometry, bond angles refer to the angles formed between bonding domains, which include both bonded atoms and lone pairs of electrons. The following molecular geometries exhibit various approximate bond angles:
1. Linear geometry: This occurs when there are two bonding domains around the central atom, resulting in a 180° bond angle. Examples include CO₂ and BeCl₂.
2. Trigonal planar geometry: With three bonding domains, the bond angles are approximately 120°. Molecules such as BF₃ and SO₃ exhibit this geometry.
3. Tetrahedral geometry: This geometry has four bonding domains, leading to bond angles of approximately 109.5°. CH₄ and NH₃ are examples of molecules with tetrahedral geometry.
4. Trigonal bipyramidal geometry: In this case, there are five bonding domains, resulting in bond angles of 90° and 120°. Examples include PCl₅ and SF₄.
5. Octahedral geometry: With six bonding domains, octahedral molecules have bond angles of 90°. Molecules like SF₆ and Cr(CO)₆ exhibit this geometry.
These bond angles can be affected by the presence of lone pairs, which create deviations from ideal bond angles. For instance, water (H₂O) has two lone pairs and a bent geometry, resulting in a bond angle of approximately 104.5° instead of the expected 109.5° in a perfect tetrahedral arrangement.
Hence, 90° ,109.5°, 120°, 180° are all the approximate bond angles.
To know more about bond angles, refer to the link below:
https://brainly.com/question/13751116#
#SPJ11
Complete Question:

A sphere of radius 0.457 m, temperature 32.2 ∘
C, and emissivity 0.924 is located in an environment of temperature 82.9 ∘
C. At what rate does the sphere (a) emit and (b) absorb thermal radiation? (c) What is the sphere's net rate of energy exchange? (a) Number (b) Number Units Units
Answers
a) The sphere emits thermal radiation at a rate of 139.75 Watts.
b) The sphere absorbs thermal radiation at a rate of 37.66 Watts.
c) The sphere's net rate of energy exchange is 102.09 Watts.
What are the rates of thermal radiation emission, absorption, and net energy exchange for the sphere?To calculate the rates of thermal radiation emission and absorption, we can use the Stefan-Boltzmann law, which states that the rate of thermal radiation emitted or absorbed by an object is proportional to its surface area, temperature, and the Stefan-Boltzmann constant.
a) The rate of thermal radiation emitted by the sphere can be calculated using the formula:
Emitting Rate = emissivity * surface area * Stefan-Boltzmann constant * (\(temperature^4 - environment\ temperature^4\))
Plugging in the given values:
Emitting Rate = \(0.924 * (4\pi * (0.457)^2) * 5.67 \times 10^{-8} * ((32.2 + 273.15)^4 - (82.9 + 273.15)^4)\)
Emitting Rate ≈ 139.75 Watts
b) The rate of thermal radiation absorbed by the sphere can be calculated in a similar way but using the environment temperature as the object's temperature:
Absorbing Rate = emissivity * surface area * Stefan-Boltzmann constant * (\(environment\ temperature^4 - temperature^4\))
Plugging in the given values:
Absorbing Rate = \(0.924 * (4\pi * (0.457)^2) * 5.67 \times 10^{-8} * ((82.9 + 273.15)^4 - (32.2 + 273.15)^4)\)
Absorbing Rate ≈ 37.66 Watts
c) The net rate of energy exchange is the difference between the emitting rate and the absorbing rate:
Net Rate = Emitting Rate - Absorbing Rate
Net Rate = 139.75 Watts - 37.66 Watts
Net Rate ≈ 102.09 Watts
Therefore, the sphere emits thermal radiation at a rate of 139.75 Watts, absorbs thermal radiation at a rate of 37.66 Watts, and has a net rate of energy exchange of 102.09 Watts.
Note: The units for all the rates are Watts.
Learn more about thermal radiation emission
brainly.com/question/28517392
#SPJ11
A compound is composed of 22.5% phosphorus and 77.5% chlorine. the molecular mass of the compound is 137.32 g/mol. what is the molecular formula of the compound? pcl2 pcl3 p2cl3 pcl4
Answers
The molecular formula of the compound is PCl₃ if the molecular mass of the compound is 137.32 g/mol.
An empirical formula can be described as a formula that tells about the relative ratios of different atoms in a compound.
The empirical formula for this compound containing phosphorus and chlorine can be calculated as follows;
As the compound is composed of 22.5% phosphorous and 77.5% chlorine, therefore;
Phosphorus = (22.5/100) × 137.32 = 0.225 × 137.32 = 30.897
Chlorine = (77.5/100) × 137.32 = 0.775 × 137.32 = 106.423
Now we can find the moles of each atom by using their molar mass as follows;
Phosphorus = 30.897 ÷ 30.97 = 0.998 moles
Chlorine = 106.423 ÷ 35.453 = 3.002 moles
Now dividing each mole value by the smallest determined number of moles (0.998) as follows;
Phosphorus = 0.998 / 0.998 = 1 mole
Chlorine = 3.002 / 0.998 = 3.008 ≈ 3 moles
Therefore the molecular formula of the compound will be PCl₃.
To learn more about the empirical formula; click here:
https://brainly.com/question/28080770
#SPJ4
Answer:
b
Explanation:
10 points for this problem that i need help with
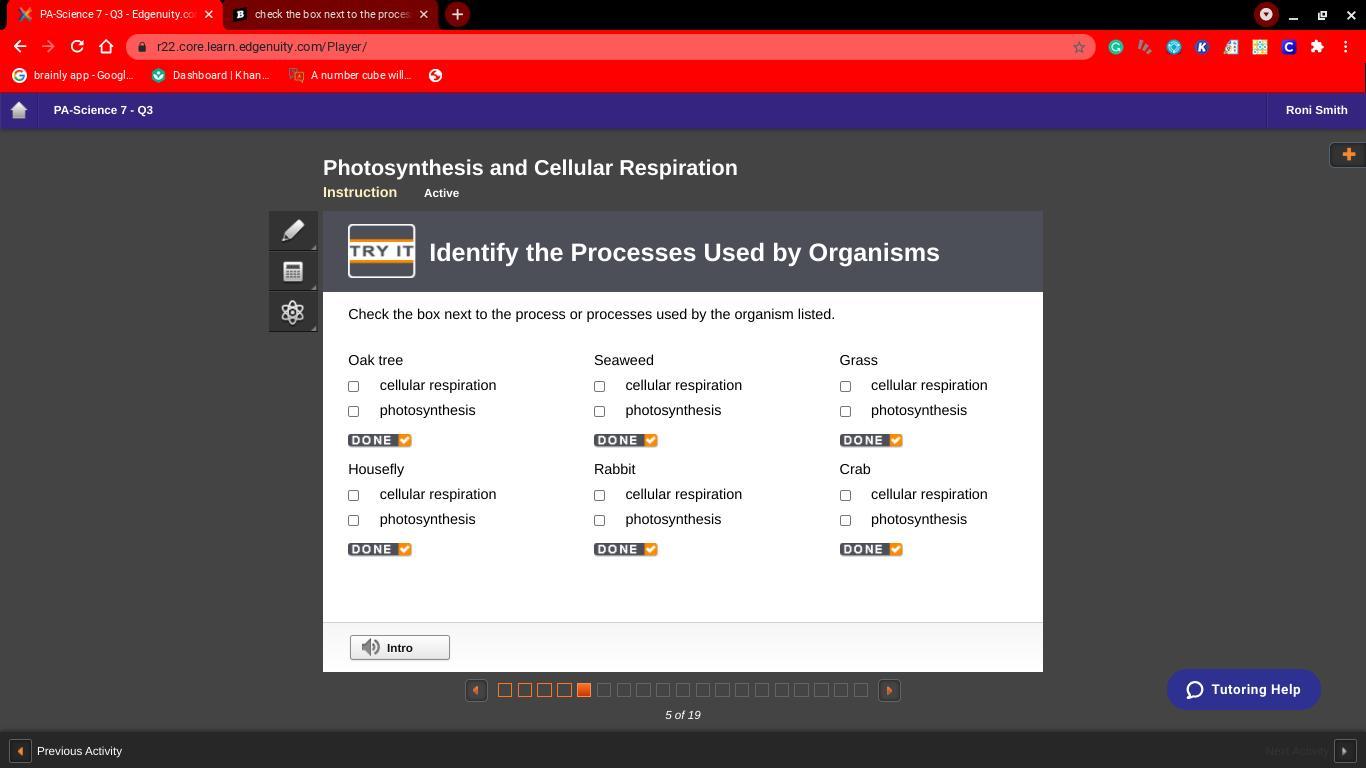
Answers
Answer:
house fly = cellular rabbit = cellular crab = cellular
Explanation:
Which statement best explains a way in which a cell gets rid of wastes?

Answers
Answer:
I would say B but you don't have to take my word for it
Answer:
D
Explanation:
What is the [H+] if the pH of a
solution is 3.20?
![What is the [H+] if the pH of asolution is 3.20?](https://i5t5.c14.e2-1.dev/h-images-qa/contents/attachments/D9ynvM4XzDDA5j7zj22DN0LAwblKnHuA.png)
Answers
Given: pH = 3.20
Solution
3.20 = - log[H+]
Divide by - 1
-3.20 = log[H+]
Take the antilog of both sides
10^-3.20 = [H+]
[H+]= 6.30 * 10^-4 moles/L
Identify the type of energy this object possesses. A girl roller-skating Kinetic energy Potential energy
Answers
A girl roller-skating has kinetic energy.
Kinetic energy is the energy of motion. It is the energy possessed by an object due to its movement. In this case, the girl roller-skating has kinetic energy because she is moving.
Potential energy is the energy an object possesses due to its position or configuration. It is the energy an object has stored within it, ready to be released. An object at rest has potential energy because it has the potential to be set in motion and does work.
So, in this case, the girl roller-skating has kinetic energy because she is moving, and not potential energy because she is not at rest.
To learn more about kinetic energy, check out https://brainly.com/question/24933254
1
Which of the following parts of Dalton's Atomic Theory IS supported by
Thomson's and Rutherford's model?
Everything is made of atoms
Electrons are larger than atoms
Atoms are indivisible
Atoms take the form of a solid sphere
Answers
Answerfirst one
Explanation:
The pressure in a car tire is 198 kPa at 27C. After a long drive, the pressure is 225 kPa.
What is the temperature of the air in the tire? Assume that the volume is constant.