Which of the following defines the wavelength of a wave? A. the distance between peaks of a wave B. the speed that a wave is passed from particle to particle C. the loudness of waves that travel through the air D. the height of a wave between peak and trough
Answers
Answer:
D.the wavelength of a wave is the distance between two successive peaks or two successive troughs or a peak and trough
Answer:
My answer to the question is wavelength is the distance between two successful cress or successful troughs of a wave. which will be option A.
Related Questions
molecular, complete, and net ionic equation of 1. ammonium nitrate and calcium hydroxide react 2. ammonium phosphate and manganese (IV) sulfate are mixed 3. iron (II) chloride + ammonium phosphate are mixed 4. sodium hydroxide + copper (II) nitrate are mixed
Answers
The net ionic equations shows the species that have undergone cahnge in the reaction.
What is the net ionic equation?For number 1;
Molecular Equation: NH4NO3 + Ca(OH)2 -> 2NH4OH + Ca(NO3)2
Complete Ionic Equation: 2NH4+ + 2NO3- + Ca2+ + 2OH- -> 2NH4+ + 2OH- + Ca2+ + 2NO3-
Net Ionic Equation: 2OH- -> H2O
For number 2;
Molecular Equation: 2(NH4)3PO4 + 3MnSO4 -> (NH4)2SO4 + Mn3(PO4)2
Complete Ionic Equation: 6NH4+ + 2PO43- + 3Mn2+ + 3SO42- -> 2NH4+ + 2SO42- + Mn3(PO4)2
Net Ionic Equation: 2PO43- + 3Mn2+ -> Mn3(PO4)2
For number 3;
Molecular Equation: 3FeCl2 + 2(NH4)3PO4 -> Fe3(PO4)2 + 6NH4Cl
Complete Ionic Equation: 6Fe2+ + 6Cl- + 2NH4+ + 2PO43- -> Fe3(PO4)2 + 6NH4+ + 6Cl-
Net Ionic Equation: 2Fe2+ + 2PO43- -> Fe3(PO4)2
For number 4;
Molecular Equation: 2NaOH + Cu(NO3)2 -> 2NaNO3 + Cu(OH)2
Complete Ionic Equation: 2Na+ + 2OH- + Cu2+ + 2NO3- -> 2Na+ + 2NO3- + Cu(OH)2
Net Ionic Equation: 2OH- + Cu2+ -> Cu(OH)2
Learn more about net ionic equation:https://brainly.com/question/13887096
#SPJ4
How many femtometers are in 540. nanometers?
a. 5.40 x 10^-4
b. 5.40 x 10^26
C.
5.40 x 10^8
d. 5.40 x 10^-6
e.
5.40 x 10^-8
Answers
Matter are anything that is made up of atoms. The quantity of matter can be observed only on the basis of mass and volume calculation. Therefore, the correct option is option A.
What is matter?Matter is a substance that has some mass and can occupy some volume. The matter is mainly used in science. Matter can be solid, liquid or gas.
Matter is anything that is made up of atoms. Anything around us that can be physically seen and touched are matter. Ice, water and water vapors are example of matter.
So as we saw that matter has some mass so mass can be measured in gram only. Mass can also be represented as number of molecules. We also saw that matter occupy some volume and that volume is measured only in liter. l nm = 1.0 × 10⁻⁶ fm
540 nanometers = 540 × 1.0 × 10⁻⁶ fm
= 5.40 x 10⁻⁴fm
Therefore, the correct option is option A.
To learn more about matter, here:
https://brainly.com/question/4562319
#SPJ1
which of the following is an indicator of a chemical reaction?
A. two different compounds mixing and remaining seperate.
B. changing states of matter (solid to liquid).
C. decreasing in size.
D. increasing in tempurature
Answers
Answer:
option . D
Increase in temperature
is an indicator of a chemical reaction
hope it helps
Answer:
answer is D
Explanation:
some signs of a chemical change are a change in colour and the formation of bubbles.
the five conditions of chemical change: colour change, formation of precipitate, formation of a gas ,odor change, temperature change.
I think it will use for you
If a mineral fizzes when it comes into contact with acetic acid, it is probably composed of calcium carbonate (CaCO3).
True
False
Answers
The given statement, "If a mineral fizzes when it comes into contact with acetic acid, it is probably composed of calcium carbonate (CaCO₃)" is true because When a mineral fizzes upon contact with acetic acid, it is a strong indication that the mineral is composed of calcium carbonate (CaCO₃).
The fizzing occurs due to the release of carbon dioxide gas (CO₂) during a chemical reaction between the acid and the carbonate compound. Calcium carbonate is a common mineral found in various forms such as limestone, marble, and chalk. It is insoluble in water but reacts readily with acids, including acetic acid, which is the main component of vinegar.
The reaction between calcium carbonate and acetic acid can be represented by the following equation:
CaCO₃ + 2CH₃COOH → Ca(CH₃COO)₂ + H₂O + CO₂
In this reaction, calcium carbonate reacts with acetic acid to form calcium acetate, water, and carbon dioxide gas. The release of carbon dioxide gas is responsible for the fizzing observed when the mineral comes into contact with the acid.
Therefore, if a mineral fizzes when it encounters acetic acid, it is likely composed of calcium carbonate (CaCO₃). This fizzing reaction is often used as a simple and effective test to identify the presence of calcium carbonate in minerals or rocks.
Learn more about calcium carbonate at https://brainly.com/question/30594488
#SPJ11
Enter the names for the elements in each of the following formulas of compounds used in health and medicineantacid, Mg(OH)2 Spell out the names of the elements separated by commas.
Answers
Answer:
\(Magnesium,\text{ Oxygen and Hydrogen}\)Explanation:
Here, we want to spell out the names of the elements present in the compound
There are three elements in the given compound
We have Mg, O and H
The names can be obtained from the periodic table of element
We have;
Mg - Magnesium
O - Oxygen
H- Hydrogen
Finding evidence to support or disprove
is
how science advances
Answers
Answer:
Scientific investigations discover evidence that helps science advance, and the purpose of scientific investigations generally is to test hypotheses. Finding evidence to support or disprove hypotheses is how science advances.
Explanation:
If a container has a volume of 654 mL at 234K, what is the temperature when the volume is 123 mL?
K (round answer to the nearrest whole number)
Blank 1: 194
Blank 2:
Answers
The temperature when the volume is 123 mL is 44K.
Using the combined gas law formula:
(P₁V₁)/T₁ = (P₂V₂)/T₂
We can rearrange the formula to solve for T₂:
T₂ = (P₂V₂*T₁)/(P₁V₁)
We are given V₁ = 654 mL and T₁ = 234K. We also know that the container is sealed, so the pressure remains constant. Therefore, we can simplify the formula to:
T₂ = (V₂*T₁)/V₁
Plugging in V₂ = 123 mL, we get:
T₂ = (123 mL * 234K)/654 mL
T₂ = 44 K (rounded to the nearest whole number)
Therefore, the temperature when the volume is 123 mL is 44K.
There are several gas laws that relate the properties of gases to each other. Here are some of the most common gas laws and their formulas:
Boyle's Law: This law relates the pressure and volume of a gas. It states that the product of the pressure and volume of a gas is constant at a constant temperature.
Formula: P1V1 = P2V2
Where P1 and V1 are the initial pressure and volume, and P2 and V2 are the final pressure and volume.
Charles's Law: This law relates the volume and temperature of a gas. It states that the volume of a gas is directly proportional to its absolute temperature at a constant pressure.
Formula: V1/T1 = V2/T2
Where V1 and T1 are the initial volume and temperature, and V2 and T2 are the final volume and temperature.
Gay-Lussac's Law: This law relates the pressure and temperature of a gas. It states that the pressure of a gas is directly proportional to its absolute temperature at a constant volume.
Formula: P1/T1 = P2/T2
Where P1 and T1 are the initial pressure and temperature, and P2 and T2 are the final pressure and temperature.
Avogadro's Law: This law relates the volume and amount (in moles) of a gas. It states that equal volumes of gases at the same temperature and pressure contain equal numbers of molecules.
Formula: V1/n1 = V2/n2
Where V1 and n1 are the initial volume and amount of gas, and V2 and n2 are the final volume and amount of gas.
Visit here to learn more about volume brainly.com/question/28058531
#SPJ11
Use the Rydberg equation to find the wavelength (in nm) of the photon absorbed when an electron in an H atom undergoes a transition from n = 1 to n = 3.
Answers
The wavelength of the photon absorbed when an electron in a hydrogen atom undergoes a transition from n = 1 to n = 3 is 1215 nm.
The Rydberg equation is given by:
1/λ = R(1/n₁² - 1/n₂²)
where λ is the wavelength of the photon absorbed or emitted, R is the Rydberg constant (1.0974 x 10⁷ m⁻¹), and n₁ and n₂ are the initial and final energy levels of the electron.
In this case, the electron in a hydrogen atom is undergoing a transition from n₁ = 1 to n₂ = 3, so we can substitute these values into the equation and solve for λ:
1/λ = R(1/1² - 1/3²)
1/λ = R(1 - 1/9)
1/λ = R(8/9)
λ = 1/(R(8/9))
λ = 1.215 × 10⁻⁷ m
To convert this to nanometers (nm), we multiply by 10⁹ nm/m:
λ = 1215 nm
To know more about Rydberg equation here
https://brainly.com/question/14649095
#SPJ4
Question 14 PM2.5 is defined as ________
- the mass concentration of particles in the air less than or equal to 2.5 micrometers in diameter. - the mass concentration of particles in the air equal to 2.5 micrometers in diameter. - the mass concentration of particles in the air greater than or equal to 2.5 micrometers in diameter. Question 15 Carbon dioxide (CO2) is a criteria air pollutant. - True - False Question 16 Roughly percent of emissions of carbon monoxide in Santa Clara County come from mobile sources (select the choice closest to the correct answer). - 50 - 75 - 25 Question 17
The term "photochemical smog" is most synonymous with which of the following criteria air pollutants? - lead (Pb) - carbon monoxide (CO) - sulfur dioxide ( SO2) - ozone (O3) Question 18 "Attainment" of ambient air quality standards requires that measured concentrations at all monitoring stations within an air district are below ambient air standards. - True - False
Answers
: PM2.5 is defined as the mass concentration of particles in the air less than or equal to 2.5 micrometers in diameter.Question 15: False, carbon dioxide (CO2) is not considered a criteria air pollutant.
Question 16: The closest answer is 50%, but the exact percentage is not provided in the question.Question 17: The term "photochemical smog" is most synonymous with ozone (O3), which is a criteria air pollutant.Question 18: True, attainment of ambient air quality standards requires that measured concentrations at all monitoring stations within an air district are below ambient air standards.
Question 14 asks about the definition of PM2.5. PM2.5 refers to particulate matter with a diameter less than or equal to 2.5 micrometers. It represents the mass concentration of particles suspended in the air, which are small enough to be inhaled into the respiratory system and can have adverse health effects.
Question 15 states whether carbon dioxide (CO2) is a criteria air pollutant. Criteria air pollutants are a set of pollutants regulated by environmental agencies due to their detrimental impact on air quality and human health. However, carbon dioxide is not considered a criteria air pollutant because it does not directly cause harm to human health or the environment in the same way as pollutants like ozone or particulate matter.
Question 16 asks about the percentage of carbon monoxide (CO) emissions from mobile sources in Santa Clara County. While the exact percentage is not provided in the question, the closest answer option is 50%. However, it is important to note that the precise percentage may vary depending on specific local conditions and emissions sources.
Question 17 inquires about the criteria air pollutant most synonymous with the term "photochemical smog." Photochemical smog is primarily associated with high levels of ground-level ozone (O3). Ozone is formed when nitrogen oxides (NOx) and volatile organic compounds (VOCs) react in the presence of sunlight, creating a hazy and polluted atmospheric condition.
Question 18 addresses the concept of "attainment" of ambient air quality standards. To achieve attainment, measured concentrations of pollutants at all monitoring stations within an air district must be below the established ambient air quality standards. This ensures that the air quality in the given area meets the required standards for protecting human health and the environment.
Learn more about mass concentration here:- brainly.com/question/23437000
#SPJ11
Which best describes the future of wind power for providing electricity in the United States?
A. promising
B. worrisome
c. problem-free
D. unlikely
Answers
Answer:
c.
Explanation:
Answer:
problem free
Explanation:
i got it right on the test
17) 4NH3 + 502 → 4NO + 6H2OIf 73 grams of NH3 are reacted and 101 grams of H20 are actually produced, what is the percentyield?
Answers
Answer
%Yield = 87.2%
Explanation
Given:
mass of NH3 reacted = 73 g
mass of H2O produced = 101 g
We know:
molar mass of NH3 = 17,031 g/mol
molar mass of water = 18.01528 g/mol
Required: % Yield
Solution:
The formula used to calculate the percentage yield is:
%Yield = (actual yield/theoretical yield) x 100
The actual yield of H2O produced = 101 g
Now lets calculate the theoretical yield first.
First find the number of moles of NH3, and use stoichiometry to find the theoretical mass of water.
n = m/M n is the moles, m is the mass and M is the molar mass.
n = 73/17,031 g/mol
n = 4.29 mol
Using the stoichiometry, there molar ratio between NH3 and H2O is 4:6
Therefore the moles of H2O = 4.29 x (6/4)
n of H2O = 6.43 mol
The theoretical mass can then be calculated:
m = n x M
m = 6.43 mol x 18.01528 g/mol
m = 115.83 g
%Yield = (101 g/115.83)*100
%Yield = 87.2%
identify and describe one common electrostatic phenomenon
Answers
Answer:
There are many examples of electrostatic phenomena, from those as simple as the attraction of the plastic wrap to one's hand after it is removed from a package to the apparently spontaneous explosion of grain silos, the damage of electronic components during manufacturing, and photocopier & laser printer operation
if evaporation occurs what will change 0.70 molar solution of CuSO4
A. Molarity will increase
B. Molarity will decrease
C. the amount of CuSO4 will decrease
D. No change in molarity or amount of salute
Answers
Answer:
A. Molarity will increase .
Explanation:
Molarity = moles of solute per litre of solution
= moles of solute / volume of solution
If evaporation occurs , volume of solution decreases and moles of solute remains constant . Hence denominator decreases and numerator remains constant .
Hence the molarity increases .
How is polypropylene commonly used?
Answers
draw the flow chart of production of silk from silk moth
please help....
no links or get reported
Answers
Too lazy to draw sorry lol.
#CarryOnLearning

I actually just looked it up via image search.
just copy by hand either of the flow charts.
if you can, use colors to fill. makes it more interesting to look at
and define a circle (or rather a few points on it) to draw it neatly. the defined points can later be transformed into the arrows.


If, for every two CO2 produced each year, three O2 are required from the atmosphere, what should be the decrease in concentration (ppm) of O2 each year
Answers
If, for every two CO₂ produced each year, three O₂ are required from the atmosphere, the decrease in concentration (ppm) of O₂ each year can be calculated as follows:
1. Determine the annual increase in CO₂ concentration in ppm. Let's say it is X ppm.
2. According to the given information, for every two CO₂ produced, three O₂ are required. So, for every X ppm increase in CO₂, we would require (3/2) * X ppm decrease in O₂.
3. Therefore, the decrease in concentration (ppm) of O2 each year would be (3/2) * X ppm.
The decrease in concentration (ppm) of O2 each year would be (3/2) times the annual increase in CO₂ concentration in ppm.
For every two CO₂ produced each year, three are required from the atmosphere. This means that as the concentration of CO₂ increases, the concentration of O2 should decrease in order to maintain a balance. By calculating the annual increase in CO₂ concentration in ppm and using the ratio of 3 O₂ to 2 CO₂, we can determine the decrease in concentration of O₂ each year.
learn more about CO₂ concentration
https://brainly.com/question/431949
#SPJ11
A hypothesis is made before the experiment is conducted.
True or fasle
Answers
It should be at beginning. A hypothesis is called an educated guess of what might happen in the experiment.
Please brainliest
Answer:
false
Explanation:
___is the transfer of heat through the movement of a fluid such as water or air.
Answers
Answer:
Convection
is the movement of heat by a fluid such as water or air. The fluid (liquid or gas) moves from one location to another, transferring heat along with it. This movement of a mass of heated water or air is called a current. Radiation is the transfer of heat by electromagnetic waves.
Given the standard enthalpy changes for the following two reactions
Given the standard enthalpy changes for the following two reactions:
(1) 2C(s) + 2H2(g)C2H4(g)...... ΔH° = 52.3 kJ
(2) 2C(s) + 3H2(g)C2H6(g)......ΔH° = -84.7 kJ
what is the standard enthalpy change for the reaction:
(3) C2H4(g) + H2(g)C2H6(g)......ΔH° = ?
Answers
The standard enthalpy change for reaction (3) is 117.1 kJ.
The standard enthalpy change for reaction (3) can be calculated by using the enthalpy changes of reactions (1) and (2) and applying Hess's Law.
To do this, we need to manipulate the given equations so that the desired reaction (3) can be obtained.
First, we reverse reaction (1) to get the formation of C2H4(g) from C2H6(g):
C2H4(g)C2H6(g) ΔH° = -52.3 kJ
Next, we multiply reaction (2) by 2 and reverse it to obtain 2 moles of C2H6(g) reacting to form 3 moles of H2(g):
2C2H6(g)2C(s) + 3H2(g) ΔH° = 169.4 kJ
Now, we add the two modified equations together:
C2H4(g)C2H6(g) ΔH° = -52.3 kJ
2C2H6(g)2C(s) + 3H2(g) ΔH° = 169.4 kJ
When adding these equations, the C2H6(g) on the left side cancels out with the C2H6(g) on the right side, leaving us with the desired reaction (3):
C2H4(g) + H2(g)C2H6(g) ΔH° = -52.3 kJ + 169.4 kJ = 117.1 kJ
Learn more about standard enthalpy here :-
https://brainly.com/question/28303513
#SPJ11
A 2.00 mL soda sample was added with HCl and diluted to 100 mL. Given the following absorbance for the standards and sample, calculate the amount of caffeine and benzoic acid in the given sample. benzoic acid (mg/mL Abs at 230 nm caffeine (mg/mL) Abs at 265 nm 2.0 0.171 4.0 0.134 4.0 0.354 8.0 0.291 6.0 0.543 12.0 0.487 8.0 0.736 16.0 0.670 10.0 0.980 20.0 0.915 (6) Determine the concentration of benzoic acid and caffeine in the analyzed sample. (5). Why is acid (HCl) added to the sample prior to the analysis?
Answers
From the standard curve, the absorbance of the 2.0 mg/mL solution is 0.171, which can be expressed as A = εbc, where ε is the molar absorptivity (liters per mole per centimeter) at 230 nm, b is the path length (cm), and c is the concentration of benzoic acid (moles per liter).
The molar absorptivity (ε) can be calculated as:ε = A/bc = 0.171/(1 × 2.0 × 10^-3) = 85.5 L mol^-1 cm^-1
Using Beer-Lambert's law, the concentration of benzoic acid in the sample can be calculated as:c = A/εb = 0.065/85.5 × 1 × 10^-2 = 7.60 × 10^-5 mol/L
The concentration of benzoic acid in the sample is 7.60 × 10^-5 mol/L. The mass of benzoic acid in the sample can be calculated as:
mass = concentration × volume × molar mass= 7.60 × 10^-5 × 100 × 10^-3 × 122.12= 0.93 mg
Concentration of caffeine:
From the standard curve, the absorbance of the 10.0 mg/mL solution is 0.980, which can be expressed as A = εbc, where ε is the molar absorptivity (liters per mole per centimeter) at 265 nm, b is the path length (cm), and c is the concentration of caffeine (moles per liter).
The molar absorptivity (ε) can be calculated as:ε = A/bc = 0.980/(1 × 10.0 × 10^-3) = 98 L mol^-1 cm^-1
Using Beer-Lambert's law, the concentration of caffeine in the sample can be calculated as:c = A/εb = 0.326/98 × 1 × 10^-2 = 3.33 × 10^-4 mol/LThe concentration of caffeine in the sample is 3.33 × 10^-4 mol/L.
The mass of caffeine in the sample can be calculated as:mass = concentration × volume × molar mass= 3.33 × 10^-4 × 100 × 10^-3 × 194.19= 6.47 mg
The mass of caffeine in the sample is 6.47 mg.
Learn more about molar absorptivity of at
https://brainly.com/question/32116893
#SPJ11
Can someone pls help me pls

Answers
Answer:
Figure 2 is a model of an atom
4. A photon has an energy of 2.93x10^-25 Jouls. What is the
frequency? What is the wavelength in nm?
Answers
Answer:
Hope this answer helps
Explanation:

help please,just the answers which student

Answers
Answer:
Students A, B, and E
Explanation:
Student A is playing in the lab, student B is sniffing the flask, you're supposed to waft, and student E is eating while working.
alculate the ph of a buffer solution obtained by dissolving 17.0 g of kh2po4(s) and 30.0 g of na2hpo4(s) in water and then diluting to 1.00 l.ph
Answers
The pH of a buffer solution can be calculated using the Henderson-Hasselbalch equation, which is pH = pKa + log ([A-]/[HA]). In this case, the acid is KH2PO4 and the base is Na2HPO4. The pH of the buffer solution is 7.43.
First, we need to find the pKa values for the acid and base. The pKa for KH2PO4 is 7.2 and for Na2HPO4 is 12.4.
Next, we need to find the concentrations of the acid and base. To do this, we can use the molar mass of each compound and divide the mass by the total volume of the solution.
For KH2PO4: molar mass
= 136.09 g/mol, mass
= 17.0 g, volume = 1.00 L
Concentration of KH2PO4
= 17.0 g / 136.09 g/mol / 1.00 L
= 0.125 M
For Na2HPO4:
molar mass
= 141.96 g/mol, mass
= 30.0 g, volume
= 1.00 L
Concentration of Na2HPO4 = 30.0 g / 141.96 g/mol / 1.00 L
= 0.211 M
Now, we can substitute these values into the Henderson-Hasselbalch equation.
pH = 7.2 + log (0.211 M / 0.125 M)
= 7.2 + log (1.688)
= 7.2 + 0.226
= 7.43
Therefore, the pH of the buffer solution is 7.43.
To know more about buffer solution visit:-
https://brainly.com/question/31367305
#SPJ11
Study the image.
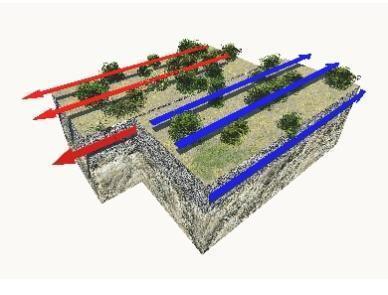
Which phrase describes this plate boundary?
does not occur in oceans
may form rift valleys
is a type of convergent boundary
is a region where earthquakes occur
Answers
The phrase that describes the plate boundary shown is D. is a region where earthquakes occur .
What is the plate boundary shown ?A transform boundary is the name given to this tectonic plate boundary. Two or more plates slip past one another in such a border. This shift is typically accompanied by some highly active earthquakes.
At a transform plate border, the grinding motion between the plates causes shallow earthquakes, significant lateral rock displacement, and a wide zone of crustal deformation. Essentially While no new crust is produced, subducted, or formed, and no volcanoes are formed, the fault produces earthquakes.
Find out more on plate boundaries at https://brainly.com/question/22005072
#SPJ1
How long did the Cambrian period last for?
Answers
Answer: 541 million to 485.4 million years ago
Explanation:
My car has an internal volume of 12,000 L. If I drive my car into the river and it implodes, what will be the volume of the gas when the pressure goes from 1.0 atm to 1.4 atm?
Answers
The volume of gas when the pressure goes from 1.0 atm to 1.4 atm is 8,571.43 L.
When a car is driven into the river, it will implode due to the change in pressure. We are to calculate the volume of gas when the pressure goes from 1.0 atm to 1.4 atm if the internal volume of the car is 12,000 L.In order to solve the problem, we will use the combined gas law equation. The equation is given as follows;P1V1/T1 = P2V2/T2where P1 is the initial pressure, V1 is the initial volume, T1 is the initial temperature, P2 is the final pressure, V2 is the final volume, and T2 is the final temperature.We will assume that the initial temperature and final temperature are constant, and therefore, we can cancel them from the equation. Thus, the equation becomes;P1V1 = P2V2We can rearrange the equation to solve for V2 as follows;V2 = (P1V1)/P2Substituting the given values, we get;V2 = (1.0 atm * 12,000 L)/1.4 atmV2 = 8,571.43 L.
For more questions on pressure
https://brainly.com/question/24719118
#SPJ8
Using a Punnett square, what genotypes would you expect in the F2 offspring?
F1 generation
F2 (option A)
F2 (option B)
Father
Father
Father
A
A
A
A
A
a
Mother
Mother
Mother
a
Аа
«Аа
a
Аа
Аа
А
AA
Аа
a
Аа
Аа
a
Аа
Aai
aa
a
А
Answers
Answer:
can you answer mine i will help you! please
Explanation:
How many molecules are in 7.32 moles of sulfur dioxide?
Answers
Answer:
4.41 × 10²⁴ molecules SO₂
General Formulas and Concepts:
Atomic Structure
CompoundsMolesStoichiometry
Using Dimensional AnalysisExplanation:
Step 1: Define
[Given] 7.32 moles SO₂
[Solve] molecules SO₂
Step 2: Identify Conversions
Avogadro's Number - 6.022 × 10²³ atoms, molecules, formula units, etc.
Step 3: Convert
[DA] Set up: \(\displaystyle 7.32 \ moles \ SO_2(\frac{6.022 \cdot 10^{23} \ molecules \ SO_2}{1 \ mol \ SO_2})\)[DA] Multiply [Cancel out units]: \(\displaystyle 4.4081 \cdot 10^{24} \ molecules \ SO_2\)Step 4: Check
Follow sig fig rules and round. We are given 3 sig figs.
4.4081 × 10²⁴ molecules SO₂ ≈ 4.41 × 10²⁴ molecules SO₂
Answer:
4.41 × 10²⁴ molecules SO₂
Explanation:
The Person is right
a cup of hot tea has blank energy
Answers
Answer: thermal energy
Explanation: Thermal energy is what we call energy that comes from heat.