Answers
I think Sr
Hope this help!
Related Questions
Write the name and the symbol of the element that satisfies the following conditions:

Answers
Answer:
Sulphur(S).......Answer:
Argon Ar is the element that satisfies the following condition
A chemist wants to make a 17.85 %(m/m) solution of NaCl using a only 50.0 g of the salt. How much water is needed to make the solution? Numerical answer only. No units.
Answers
The mass percentage is an important method which is used to calculate the concentration of a solution. The amount of water needed to add in order to make 17.85 % NaCl solution is 230.1 g.
What is mass percentage?The mass percentage of a component in a solution is defined as the mass in grams of that component present per 100 g of the solution. The term mass percentage is denoted as w/w. It is used to calculate the concentration of a binary solution.
Mass percentage = Mass of the component in the solution / Total mass of solution × 100
17.85 = 50.0 / 50.0 + x × 100
0.1785 (50.0 + x) = 50.0
8.925 + 0.1785 x = 50.0
0.1785 x = 41.075
x = 230.1 g
Thus the amount of water added to make the solution is 230.1 g.
To know more about mass percentage, visit;
https://brainly.com/question/27429978
#SPJ2
A gas has a volume of 550 mL at a temperature of -55 °C. The volume of the gas at 30 °C is
Blank 1:
mL.
Answers
The combined gas law equation is:
(P1 * V1) / (T1) = (P2 * V2) / (T2)
The volume of the gas at 30 °C is approximately 760.67 mL.
To determine the volume of the gas at 30 °C, we can use the combined gas law equation, which relates the initial and final conditions of temperature and volume for a gas.
The combined gas law equation is:
(P1 * V1) / (T1) = (P2 * V2) / (T2)
Where:
P1 and P2 are the initial and final pressures, respectively
V1 and V2 are the initial and final volumes, respectively
T1 and T2 are the initial and final temperatures in Kelvin, respectively
We need to convert the temperatures from Celsius to Kelvin by adding 273.15 to each value.
Given:
V1 = 550 mL
T1 = -55 °C = 218.15 K
T2 = 30 °C = 303.15 K
Assuming the pressure remains constant, we can rearrange the equation to solve for V2:
V2 = (P1 * V1 * T2) / (P2 * T1)
Since the pressure is not specified in the problem, we can assume it remains constant, allowing us to cancel out the pressure terms. Thus, the final equation becomes:
V2 = (V1 * T2) / T1
Plugging in the given values:
V2 = (550 mL * 303.15 K) / 218.15 K
Simplifying the calculation, we find:
V2 ≈ 760.67 mL
Therefore, the volume of the gas at 30 °C is approximately 760.67 mL.
For more question on gas law
https://brainly.com/question/27870704
#SPJ8
is Al+CuCl2 a spontaneous reaction?
Answers
Answer:
No, it isn't a spontaneous reaction.
Explanation:
A spontaneous reaction is a reaction that requires no outside intervention to start.
All chemical reactions require energy.
mark brainliest please!
For the diprotic weak acid H2A, a1=3.2×10−6 and a2=6.1×10−9 .
What is the pH of a 0.0750 M solution of H2A ?
What are the equilibrium concentrations of H2A and A2− in this solution?
Answers
In the first dissociation of H2A:
molarity H2A(aq)↔ (HA)^-(aq) + H^+(aq)
initial 0.05 m 0 m 0 m
change -x +x +x
equilibrium 0.05-x x x
we can neglect X in [H2A] as it so small compared to the 0.05
so by substitution in Ka equation:
Ka1 = [HA][H] / [H2A]
2.2x10^-6 = X^2/0.05
X = √(2.2x10^-6)*(0.05)= 1.1x10^-7
X= 3.32x10^-4 m
∴ [H2A] = 0.05 - 3.32x10^-4 = 0.0497 m
[HA] = 3.32x10^-4 m
[H] = 3.32x10^-4 m
the second dissociation of H2A: when ka2 = 8.2x10^-9
HA-(aq) ↔ A^2- (aq) + H+(aq)
at equilibrium 3.32x10^-4 y 3.32x10^-4
Ka2 = [H+][A^2-] / [HA]
8.2x10^-9 = Y(3.32x10^-4)/(3.32x10^-4)
∴y = 8.2x10^-9 m
∴[A] = 8.2x10^-9 m
PH= -㏒[H+]
= -㏒(3.32x10^-4)= 3.479
[A]=8.2x10^-9 m
[H2A] = 0.0497 ≈ 0.05 m
I understand how a change in the size of the moon jellies' resource population can change the number of births in the moon jelly population.
Responses
Explain your answer choice.
Answers
A change in the size of the moon jellies' resource population can change the number of births in the moon jelly population because the big size of the resources can produce more births.
How do moon jellies reproduce?When there is more energy storage molecules present in the moon jellies, they can reproduce more, in more births. Fewer deaths would also lead to the jelly population increasing. The sea turtle population, and the moon jellies consumer population is also decreased.
There must be a change to the birth rate or the death rate in the moon jelly population. Within a population, organisms are born and dying continuously. If the number of births and deaths in a given time interval are equal, then the population size will remain stable.
So we can conclude that a large population of resources will lead to more births.
Learn more about jellies here: https://brainly.com/question/25630111
#SPJ1
A food stall owner was preparing dough for making bhatura. He added a pinch of yeast and sugar to the dough and left it in a warm place. After a few hours, the dough had risen. There was our sell too.
1. Why did the dough rise?
2. Why did the dough smell sour?
3. Why was sugar added to the dough? 4.What would have happened if the dough was kept in the refrigerator, soon after it prepared?
Answers
Explanation:
yeast and sugar was present
This chart shows global energy usage for the year 2005. Solar, 0.5% Hydroelectric, 3% Wind, 0.3% Biomass Geothermal, 0.2% Nuclear Oil 379 Natural gas 23% Need an extra pair of e Get writing feedback fri real tutor Submit a review Coal Use the chart to answer the following questions. (8 points) A. What total percent of energy came from fuels that emitted greenhouse gases?
Answers
Approximately 60.9% of the total energy in 2005 came from fuels that emitted greenhouse gases. This signifies a significant contribution to global greenhouse gas emissions and highlights the importance of transitioning to cleaner and more sustainable energy sources to mitigate climate change impacts.
To determine the total percent of energy that came from fuels emitting greenhouse gases, we need to consider the energy sources listed in the chart that are known to produce greenhouse gas emissions. In this case, those would be oil, natural gas, and coal.
From the chart, we see that the percentages for these three energy sources are:
Oil: 37.9%
Natural gas: 23%
Coal: Not specified
Although the percentage for coal is not mentioned in the given information, it is a known fact that coal combustion releases greenhouse gases, including carbon dioxide (CO2). Therefore, we can assume that coal is among the fuels emitting greenhouse gases.
Adding up the percentages for oil and natural gas, we have:
37.9% (oil) + 23% (natural gas) = 60.9%
Therefore, approximately 60.9% of the total energy in 2005 came from fuels that emitted greenhouse gases. This signifies a significant contribution to global greenhouse gas emissions and highlights the importance of transitioning to cleaner and more sustainable energy sources to mitigate climate change impacts.
For more question on energy
https://brainly.com/question/30745996
#SPJ11
The table shows the temperature and pressure of five-liter samples of four different gases. Which two gas samples do not have the
same number of molecules?
Answers
Answer:He2 & O2
Explanation:
Answer:
Answer:A
Explanation:
Calculate the cell potential for the galvanic cell in which the given reaction occurs at 25 °C, given that [Sn2+]=0.0624 M, [Fe3+]=0.0437 M, [Sn4+]=0.00655 M, and [Fe2+]=0.01139 M. Standard reduction potentials can be found in this table.
Sn2+(aq)+2Fe3+(aq)↽−−⇀ Sn4+(aq)+2Fe2+(aq)
So far my incorrect answers have been:
0.28
0.798
0.178
0.142
0.881
0.61
and 0.812
Answers
Answer:
The cell potential for the given galvanic cell is 0.188 V.
Explanation:
To calculate the cell potential, we can use the Nernst equation:
Ecell = E°cell - (RT/nF)ln(Q)
where E°cell is the standard cell potential, R is the gas constant (8.314 J/mol·K), T is the temperature in Kelvin (25°C = 298 K), n is the number of moles of electrons transferred (in this case, n = 2), F is the Faraday constant (96,485 C/mol), and Q is the reaction quotient.
First, we need to write the half-reactions and their standard reduction potentials:
Sn4+(aq) + 2e- → Sn2+(aq) E°red = 0.15 V
Fe3+(aq) + e- → Fe2+(aq) E°red = 0.77 V
The overall reaction is the sum of the half-reactions:
Sn2+(aq) + 2Fe3+(aq) → Sn4+(aq) + 2Fe2+(aq)
The reaction quotient Q can be expressed as:
Q = [Sn4+][Fe2+]^2 / [Sn2+][Fe3+]^2
Substituting the given concentrations, we get:
Q = (0.00655)(0.01139)^2 / (0.0624)(0.0437)^2 = 0.209
Now we can calculate the cell potential:
Ecell = 0.15 V + 0.0592 V log([Fe2+]^2/[Fe3+]) + 0.0592 V log([Sn4+]/[Sn2+])
= 0.15 V + 0.0592 V log(0.01139^2/0.0437^2) + 0.0592 V log(0.00655/0.0624)
= 0.188 V
Therefore, the cell potential for the given galvanic cell is 0.188 V.
The cell potential for the given galvanic cell in which the given reaction occurs at 25 °C is 0.188 V.
How to the cell potential of galvanic cell?To find the cell potential, we take the Nernst equation:
Ecell = E°cell - (RT/nF)ln(Q)
In which R is the gas constant (8.314 J/mol·K) and E° cell is the standard cell potential.
T temperature in Kelvin (25°C = 298 K), and n is the number of moles of electrons transferred (n = 2), Q is the reaction quotient and F is the Faraday constant (96,485 C/mol).
Firstly, write the half-reactions and then their standard reduction potentials:
Sn⁴⁺(aq) + 2e⁻ → Sn²⁺(aq) E°red = 0.15 V
Fe³⁺(aq) + e⁻ → Fe²⁺(aq) E°red = 0.77 V
The overall reaction is the sum of the half-reactions:
Sn²⁺(aq) + 2Fe³⁺(aq) → Sn⁴⁺(aq) + 2Fe²⁺(aq)
The Q reaction quotient can be written as:
Q = [Sn⁴⁺][Fe²⁺]² ÷ [Sn²⁺][Fe²⁺]²
Substituting the given concentrations, we observe:
Q = (0.00655)(0.01139)² ÷ (0.0624)(0.0437)² = 0.209
Next, we can find the cell potential:
Ecell = 0.15 V + 0.0592 V log([Fe²⁺]²/[Fe³⁺]) + 0.0592 V log([Sn⁴⁺]/[Sn²⁺])
= 0.15 V + 0.0592 V log(0.01139²÷0.0437²) + 0.0592 V log(0.00655÷0.0624)
= 0.188 V
Thus, the cell potential for the given galvanic cell is 0.188 V.
Learn more about cell potential, here:
https://brainly.com/question/29719917
#SPJ2
What is the mass (in g) of a solid piece of iron which has a specific heat of 0.449 J/g°C if when it absorbed 948.0 J of heat the temperature rose from 24.0°C to
82.1°C? Give your answer in 3 sig figs.
Answers
Answer:
Explanation:
We can use the formula:
q = mcΔT
where q is the heat absorbed, m is the mass, c is the specific heat, and ΔT is the change in temperature.
Given:
specific heat of iron (c) = 0.449 J/g°C
initial temperature (T1) = 24.0°C
final temperature (T2) = 82.1°C
heat absorbed (q) = 948.0 J
Substituting the given values into the formula, we get:
q = mcΔT
948.0 J = m(0.449 J/g°C)(82.1°C - 24.0°C)
948.0 J = m(0.449 J/g°C)(58.1°C)
m = 948.0 J ÷ (0.449 J/g°C × 58.1°C)
m = 33.1 g
Therefore, the mass of the iron piece is 33.1 g (to three significant figures)
Consider the reaction 3X + 2Y -> 5C + 4D
With excess Y, how many moles of X are needed to produce 25.00 moles of D?
Answers
Answer:
The correct answer is 18.75 moles X.
Explanation:
We first start with the idea that we have 25.00 moles of D. Next, we must look at the relationships in the balanced equation to find that for every 4 moles of D produced, 3 moles of X are reacted (since it is given that there is excess Y and thus X is the limiting reagent). From this, we can do the following calculation:
25.00 moles D * (3 moles X/4 moles D) = 18.75 moles X
Therefore, your answer is 18.75 moles X.
Hope this helps!
Which one is it I don’t know

Answers
Using the prefix method for covalent compounds, the names of the given compounds are shown below:
LIF: Lithium fluoride
Cl2O7: Dichlorine heptoxide
N2O3: Dinitrogen trioxide
SF6: Sulfur hexafluoride
Na3PO4: Sodium phosphate
What are covalent compounds?A covalent bond is described as a chemical bond that involves the sharing of electrons to form electron pairs between atoms.
The properties of covalent compounds includes:
The boiling/melting points of covalent compounds are low.covalent compounds are soft in nature and relatively flexible.covalent compounds do not possess electrical conductivity.Learn more about covalent compounds at:
https://brainly.com/question/3447218
#SPJ1
What is the correct equilibrium constant expression for the following reaction? 3A2 = 2B3 when the reaction started with the initial concentrations of A2 = 3 M and B3 = 2 M and continued until the equilibrium concentrations of A2 = 2.5 M and B3 = 2.5 M
Answers
Answer:
Kc = [B₃]²/[A₂]³ = 0.40
Explanation:
3A₂ ⇄ 2B₃
Given at equilibrium => [A₂] =2.5 and [B₃] = 2.5
Kc = [B₃]²/[A₂]³ = (2.5)²/(2.5)³ = (2.5)⁻¹ = 0.40
why is food coloring added to water and it turning blue is a physical change
Answers
Answer:
No new substance is formed.
Explanation:
A hereditary mutation is one that is unique to the individual organism?
True or false
Answers
Answer:
I think it is True
Explanation:
Why are atoms electrically neutral if they contain charged particles?
Answers
Answer:
Atoms are electrically neutral because they have equal numbers of protons (positively charged) and electrons (negatively charged).
Explanation:
How many moles are in 5.77 grams of calcium
Answers
Given:
Mass of calcium= 5.77 grams Number of calcium atom = \(\frac{5.77g}{40.1g}\) × ( Avogadro's number)(Avogadro's number= 6.022 × 10^23 mol\(^{-1}\)
Number of calcium atom= \(\frac{5.77g}{40.01g}\)× 6.022 × \(10^{23}\) \(mol^{-1}\)
= 8.677× 6.022 \(10^{22}\)
Therefore, number of moles that are in 5.77 grams of calcium is (8.677 × 10^22)
Learn more at: https://brainly.com/question/13314627
Calculate the mass (in grams) of chlorine (Cl2) gas sample which occupies a 2.50 L container at a pressure of 1.22 atm and temperature of 27.8°C?
Answers
Answer:Nothing
Explanation:
The answer is nothing the tempatature isnt matched with the degrees this is false
what cause a mass defect?
○A. The mass of a nucleus is larger than it should be.
○B. The mass of a nucleus cannot be accurately measured.
○C. Mass is converted to the energy binding a nucleus together.
○D. Mass is lost when a particle is released in a reaction.
Answers
Answer: Mass is converted to the energy binding a nucleus together.
Explanation: a p e x

Mass is converted to the energy binding a nucleus together cause a mass defect.Hence , Option (C) is correct.
What is Mass defect ?
The actual atomic mass is less than the predicted mass calculated by adding the masses of nucleons.
This additional mass is accounted for by binding energy that is released when a nucleus is formed.
When a nucleus is formed, some of the mass is converted to energy and this results in the mass defect.
Therefore, Mass is converted to the energy binding a nucleus together cause a mass defect. Hence , Option (C) is correct.
Learn more about mass defect here ;
https://brainly.com/question/11624098
#SPJ2
I would calculate the number of protons by?
Answers
Answer:
The number of proton in an atom is equal to the atomic number of that element. Thus, Number of protons = Atomic number ( Z )
`In this lesson we discussed how to abbreviate electron configurations using what is called noble gas configuration. So if you were writing such a configuration for phosphorus, which noble gas would you use?
Answers
Answer:
Concept: Advanced Chemistry Techniques
Use the periodic table to locate the nearest noble gas (At the far right) P is #15 and the nearest noble gas is #10 NEWhat is the critical temperature of water? What does this temperature value represent?
Answers
between cold hot so i guess regular
Answer:
374oC
critical temperature (oC)
Tubes containing water at several temperatures. Note that at or above 374oC (the critical temperature for water), only water vapor exists in the tube.
I hope this helps
Select all of the following that are combustion reactions.

Answers
Answer:
Explanation:
1,2,4,
The equations that show combustion are equations A, B and D.
What is combustion?When we talk about combustion, the idea is that the substance would be burnt in oxygen. In other words, the combustion can be taken to be an oxidation reaction. It is an oxidation reaction in the sense that the oxidation number of the substance that is reacting with the oxygen would become increased.
When we look at the equations that we have, it is quite easy to pick out among the balanced reaction equations that are shown here the ones that has to do with the burning of the substance in oxygen and a consequent rise in the oxidation number.
Learn more about combustion:https://brainly.com/question/15117038
#SPJ1
22. What is the central idea of "A Brief History of DDT"?

Answers
The central idea is to combat malaria, typhus, and other insect-borne human diseases among both military and civilian populations.
The full form of DDT is Dichlorodiphenyltrichloroethane.
DDT is an insecticide used in agriculture. The United States banned the use of DDT in 1972. Some countries outside the United States still use DDT to control mosquitoes that spread malaria.
It also was effective for insect control in crop and livestock production, institutions, homes, and gardens. DDT's quick success as a pesticide and broad use in the United States and other countries led to the development of resistance by many insect pest species.
DDT is:
known to be very persistent in the environment,will accumulate in fatty tissues, andcan travel long distances in the upper atmosphere.To know more about DDT:
https://brainly.com/question/12137640
Which is correct order of the weather observed with each cloud type from 1 to 4?
A. Rain, thunderstorm, snow, fair
B. Light rain, fair, thunderstorm, fair
C. Light rain, thunderstorm, fair, fair
D. Hail, lightning, thunderstorm, fair
Answers
Answer:i thinks its A
Explanation:but who knows
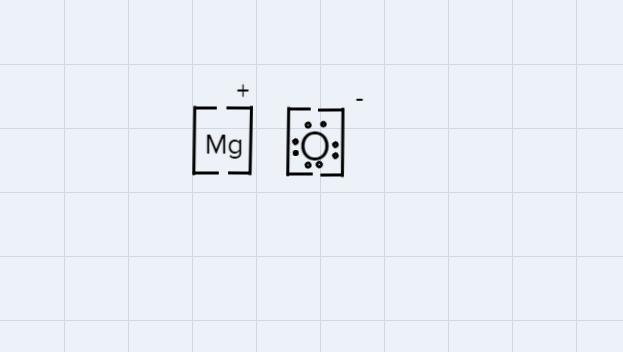
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?

Answers
Answer:
C.
Explanation:
The correct way to write the diagram of an ionic compound is:
- Use brackets by writing inside them, the symbol of each element separately.
- Draw the symbol and the number o the charge of each one, negative (-) add positive +()., in this case it is +1 for Mg (because Mg lost an electron) and -1 for O (because O won an elec).
Mg3N2 + 6H2O
--->
3 Mg(OH)2 + 2NH3
Answers
Answer:
The equation is balanced
Explanation:
There are 3Mg,2N,12H,6O and on the other side its the same amount on the other side
A naturally occurring sample of an element contains only two isotopes. The first isotope has a mass of 62.9296 amu and a natural abundance of 69.15 %. The second isotope has a mass of 64.9278 amu. You may want to reference (Page) Section 2.8 while completing this problem Part A Find the atomic mass of the element. 64.58 amu O 63.55 amu O 69.72 amu O 63.05 amu Submit Request Answer
Answers
correct answer is 63.22 amu.
Determining the atomic mass of an element requires calculating the weighted average of the masses of the two isotopes, taking into account their natural abundance. The atomic mass of an element is given by the formula:
Atomic mass = (mass of isotope 1 × natural abundance of isotope 1) + (mass of isotope 2 × natural abundance of isotope 2)
Inserting the values given in the question gives:
Atomic mass = (62.9296 amu × 0.6915) + (64.9278 amu × (1 - 0.6915))
= 43.40 amu + 19.82 amu
= 63.22 am
Therefore, the atomic mass of the element is 63.22 amu.
Read more about this on brainly.com/question/11026782
#SPJ4
HELLP ME PLSSS
The passing of heat through a material is called ________.
A. vibration
B. conduction
C. radiation
D. convectio
Answers
Answer:
B
Explanation:
conduction is the transfer of heat between objects that touch