When the beam of light hits the right or left side of the globe near the equator, what shape is the light?
Answers
Answer:
the shape of the light after it hit the right or left side of the globe near the equator is
A long oval shape..
The shape of the beam of light after striking the equator has been a long oval shape.
Globe has been the model of the earth and the incidence of light to the globe has been similar to the incidence of solar radiations on the earth. The equator has been the latitudinal ray passing from the center of the earth. The equator divides the earth into two semi-circles.
The incidence of a beam of light at the left or right side of the globe has been deflected in the shape of the oval that covers the region of the globe around the equator.
For more information about the beam of light, refer to the link:
https://brainly.com/question/9054772
Related Questions
Why does the hemisphere that is titled away from the sun experience winter?
(science)
Answers
I need help asap!!! At least with the first part

Answers
Answer:
The correct answer -
a. Cd and Pb(NO3)2
b. Redox reactions
c. Pb and Cd(NO3)2
Explanation:
This is the reaction known as the redox or reduction-oxidation reaction of metals. In this particular reaction, there are two reactants Cadmium (III) in solid-state and lead (II) nitrate in the aqueous state. At the end of this reaction, the products that we get are lead (II) in solid-state and Cadmium (III) nitrate in the aqueous state.
cadmium (s)+ lead nitrate (aq) = lead (s) + cadmium nitrate (aq)
Cd (s) + Pb2+(aq) → Pb(s) + Cd2+(aq)
Here, Oxidizing agent is Pb2+ and the reducing agent is Cd.
what is the pressure in a 20.0-l cylinder filled with 0.683 mol of nitrogen gas at 325 k?
Answers
Answer:
0.91073 atm.Explanation:
Solution 60P
Given:
Volume = 20.0 liters
Temperature = 325 K
Amount of Nitrogen = 0.683 mol
Want to know: Pressure
Explanation:
According to the ideal gas law, it is known that
P⋅V=n⋅R⋅T
Since we are looking for Pressure, we will rearrange the equation (by dividing by Volume on both sides:
P=\(\frac{nRT}{V}\)
R is the gas constant and for equations where we are working in liters and atmospheres is 0.08205746 l⋅atm mol⁻¹⋅K⁻¹
Now all we do is plug in the values and we should get out answer in atmospheres:
\(\frac{0.683 ~ X ~ 0.08205746 ~ X ~ 325 }{20.0} =\) \(0.91073\) atm pressure
Thus the pressure of cylinder is 0.91073 atm.
How many liters are in 4.5 moles of CO2 gas at STP? Pls help :)
Answers
Answer:
Explanation:
NCO 2= 4,5
VCO2= 4,5* 22,4=100,8
an atom of vanadium (z = 23) in its ground state has _____ valence electrons.
Answers
An atom of vanadium (Z = 23) in its ground state has 5 valence electrons.
Valence electrons are the electrons in the outermost energy level of an atom. In the case of vanadium, it has an electron configuration of [Ar] 4s^2 3d^3. The outermost energy level is the 4s orbital, which contains 2 electrons, and the 3d orbital, which contains 3 electrons. The total number of electrons in the outermost energy level, therefore, is 5, making them the valence electrons. Valence electrons play a crucial role in determining the chemical properties and reactivity of an element. They are involved in bonding with other atoms and determining the element's ability to form compounds.
To learn more about electrons click here: brainly.com/question/12001116 #SPJ11
What is the ionic equation for this reaction:
MgO (s) + 2HCl (aq) = MgCl2 (aq) + H2O (l)
Please let me know how you worked it out, thankyou!!
Answers
Answer:
\(MgO _{(s)} + 2H {}^{ + } _{(aq)} = Mg {}^{2 + } _{(aq)}+ H _{2} O _{(l)} \\ \)
What is the molarity of p-nitroaniline in a solution if the absorbance is 0.233 assuming a path length of 1.00 cm ? The molar absorptivity for p-nitroaniline can be found immediately before the experimental section in Experiment B. Include units in answer. Use 3 significant figures for answer.
Answers
The molarity of p-nitroaniline in a solution if the absorbance is 0.233 assuming a path length of 1.00 cm is 0.0809 M.
How to find molarity of p-nitroaniline in a solution?
The molarity is the measure of the number of moles of solute per unit volume of a solution.
The formula for molarity is:
Molarity = Moles of solute / Volume of solution (in liters)
Given data:
Absorbance = 0.233
Path length = 1.00 cm
Molar Absorptivity = 7,700 L / mol * cm (given in the experimental section)
Molar mass of p-nitroaniline = 139.11 g/mol
To calculate the molarity of p-nitroaniline in a solution, we have to first calculate the concentration of p-nitroaniline (in mol/L).
To calculate the concentration of p-nitroaniline, we have to use the Beer-Lambert law.
A = εlcwhere, A = absorbance, ε = molar absorptivity, l = path length, and c = concentration Rearranging the formula:
c = A / εlc = 0.233 / (7,700 L/mol*cm × 1.00 cm)c
= 0.0000302650 mol/L Molarity
= Moles of solute / Volume of solution (in liters)Moles of p-nitroaniline
= (Concentration × Volume of solution) / Molar mass of p-nitroaniline Moles of p-nitroaniline
= (0.0000302650 mol/L × 1000 mL) / 139.11 g/mol Moles of p-nitroaniline
= 0.00021884 mol/L
= 2.1884 × 10-4 L Molarity
= Moles of solute / Volume of solution (in liters)Molarity
= 2.1884 × 10-4 L / 1.00 L Molarity
= 0.0809 M
Thus, the molarity of p-nitroaniline in a solution if the absorbance is 0.233 assuming a path length of 1.00 cm is 0.0809 M.
#SPJ11
Learn more about p-nitroaniline:
https://brainly.com/question/17114364
Which of the following statements about alkynes is incorrect?
Alkynes are unsaturated hydrocarbons.
Alkynes have triple bonds.
Alkynes are saturated hydrocarbons.
Alkynes have carbon atoms.
Answers
Answer:
Alkynes are saturated hydrocarbons
Explanation:
Alkynes are groups of hydrocarbons that have the general formula of {Cn H2n - 2}. They have a triple carbon to carbon bond (C≡C) in their structure. Ethyne (HC≡CH) is the first member of the alkyne series.
Alkynes like alkenes are unsaturated hydrocarbons because of their triple and double carbon bonds respectively. Unsaturated hydrocarbons do not have a complete hydrogen atom in their structural composition. Hence, the statement that "Alkynes are saturated hydrocarbons" is FALSE.
PLEASE HELP ME ITS DUE IN A COUPLE OF HOURS

Answers
All of the following molecules except _________ make up a homologous series. Question 21 options: CH3CH2CH2CH3 CH3CHCHCH3 CH3CH2CH3 CH3CH2CH2CH2CH3 CH3CH2CH2CH2CH2CH3
Answers
All of the following molecules except CH₃CH₂CH₃ make up a homologous series.
What is Homologous Series ?A homologous series is a series of compound having same general formula and same functional group.
What is the general formula of alkene and alkane ?The general formula of alkane is CₙH₂ₙ₊₂
The general formula of alkene is CₙH₂ₙ
CH₃CH₂CH₃ (Propane) is the third homologous series of alkane which satisfies the general formula of alkane.
Thus from the above conclusion we can say that All of the following molecules except CH₃CH₂CH₃ make up a homologous series.
Learn more about the Homologous Series here: https://brainly.com/question/20700241
#SPJ4
C. We should only carry out terrace ......... on the sloppy land.
Answers
We should only carry out terrace farming on the sloppy land. This is the type of farming done on slope land
What is terrace farming?Terrace farming is a method of farming where steps (terraces) are built into the slopes of a hill or mountain to create flat surfaces for cultivation. This technique is commonly used in areas with steep slopes or hills where traditional flat farming is not feasible.
The terraces help to prevent soil erosion, improve water retention, and make it easier to cultivate crops. Terrace farming has been used for centuries by various civilizations around the world, including the Incas in South America and the rice farmers of Southeast Asia.
Read more on Terrace farming herehttps://brainly.com/question/20129904
#SPJ1
NO LINKS PLS HELP
Which weighs more a sealed, half-filled jar of water or that same jar after it is placed in the freezer until the water turns to ice? How do you know the answer without experimenting?
Answers
Answer:
frozen
Explanation:
I would say because when u freeze water it expands and denifys.
How many mole of a ga are preent in 50cm3 at a preure of 2kPa and a temperature of 16 degree Celiu
Answers
The mole of a gas is therefore =4×10⁻⁴mol
What is mole?One typical sort of skin development is the mole (nevi).They are frequently caused by clusters of pigment-forming cells and appear as tiny, dark brown dots (melanocytes). Most people have between 10 and 40 moles, which typically occur between the ages of 10 and 40 and may change or vanish over time. The mole (symbol: mol) is the unit of material quantity used by the International System of Units. The amount of a substance determines how many elementary entities of that substance are present in an object or sample. The mole must contain precisely 6.022140761023 elementary entities.
Is a mole a cancer and what are causes?Despite the fact that common moles are not dangerous, individuals who have more than 50 of them have a higher risk of acquiring melanoma (1). If a person notices any of the following changes in a common mole, they should inform their doctor (2): The hue shifts.
Melanocytes are the cells that produce the pigment that gives skin its natural color. Moles may darken following sun exposure, in adolescence, and during pregnancy.
Briefing:P=2K
V=50cm³
Change in dm³
=0.5dm³
T=16c
Change in Kelvin
=16+273
=289K
R=8.31mol
n=PV/RT
=2×0.5/8.31×289
=0.0004
=4×10⁻⁴ mol
To know more about Mole visit:
https://brainly.com/question/26416088
#SPJ4
、 ▼ Part C The following substances dissolve when added to water. Classify the substances according to the strongest solute-solvent interaction that will occur between the given substances and water during dissolution Drag the appropriate items to their respective bins. Not all bins may contain an item and some bins may contain multiple items View Available Hint(s) Reset Help lon-ion forces Dipole-dipole forces lon-dipole forces London dispersion forces
Answers
Ion-ion forces: occur between ions, charged particles. Dipole-dipole forces: between polar molecules. Ion-dipole forces:between ions,polar molecules. London dispersion forces: between nonpolar molecules.
To classify the substances based on the strongest solute-solvent interaction during dissolution in water, we need to consider the types of intermolecular forces involved. When a substance dissolves in water, the strength of the solute-solvent interaction is determined by the intermolecular forces present. There are four main types of intermolecular forces: ion-ion forces, dipole-dipole forces, ion-dipole forces, and London dispersion forces. Ion-ion forces: These forces occur between ions, which are charged particles. Substances that dissociate into ions when dissolved in water, such as ionic compounds like salts (e.g., NaCl), exhibit strong ion-ion interactions with water molecules due to the attraction between oppositely charged ions.
Dipole-dipole forces: These forces occur between polar molecules. Polar substances, such as alcohols (e.g., ethanol, CH3CH2OH) and organic acids (e.g., acetic acid, CH3COOH), can form strong dipole-dipole interactions with water molecules, as the positive and negative ends of the polar molecules align with the water molecule's partial charges. Ion-dipole forces: These forces occur between ions and polar molecules. Some ionic compounds that have partially charged ions, such as potassium chloride (KCl), can interact with water molecules through ion-dipole forces, leading to their dissolution in water.
London dispersion forces: These forces occur between nonpolar molecules. Nonpolar substances, such as hydrocarbons like hexane (C6H14) or cyclohexane (C6H12), can exhibit weak London dispersion forces with water molecules. However, these interactions are generally not strong enough to facilitate significant dissolution in water. By considering the nature of the substances and their intermolecular forces, we can classify them according to the strongest solute-solvent interaction that will occur during dissolution in water.
To learn more about London dispersion forces click here:
brainly.com/question/30763886
#SPJ11
In a science experiment, 10 tomato plants were given fertilizer in their water each week and 10 tomato plants were given plain water each week. All other factors were the same for all plants. At the end of each week, the height of each plant was measured. What was the independent and dependent in the experiment?
1. the height of the plant
2. the amount of water given
3. the type of plant
4. whether or not there was fertilizer in the water
Answers
Answer:
Independent: whether or not there was fertilizer in water
Dependent: height of plants
Explanation:
In an experiment, there is an independent and a dependent variable. The independent variable is what is changed in the experiment. The dependent variable is what is measured.
In this experiment, 10 tomato plants are given fertilizer in the water, while 10 other tomato plants are given regular water. Everything else is kept constant, and the heights are measured at the end of the week.
The independent variable is what is being changed. In this experiment, the only thing being manipulated is the fertilizer. One group of the plants is given fertilizer, while the other is not. Therefore, the independent variable is whether or not there was fertilizer in the water.
The dependent variable is what is being measured. In this experiment, the thing that is being measured is the height of the plants. Therefore, the dependent variable is the height of the plants.
Task 1:
Which process involves the movement of sediment?
a. Deposition
b. Weathering
c. Erosion
d. Cementing
Answers
Hope this helped
How can a fire be extinguished?
Answers
Answer:
Answer below!
Explanation:
A fire can be extinguished by many forms like fire extinguisher, but the main is water. Water can extinguish fire.
THANK U
Answer:
The preferred method for extinguishing class “A” fires is to remove the heat. Water is the most common agent, but others such as dry chemical, halon, halogenated agents and foam can be used effectively. A class “B” fire involves flammable liquid or gas.
Explanation:
Describe the structure and bonding in silicon dioxide and explain why it is a suitable material for making welding blankets.
Answers
The molecular geometry of silicon dioxide is linear and bonding in it is covalent due to which it is a suitable material for making welding blankets.
What is molecular geometry?Molecular geometry is defined as a three -dimensional arrangement of atoms which make up the molecule.It includes parameters such as bond length,bond angle and torsional angles.
It influences many properties of molecule such as reactivity,polarity color,magnetism .The molecular geometry can be determined by various spectroscopic methods and diffraction methods , some of which are infrared,microwave and Raman spectroscopy.
Learn more about molecular geometry,here:
https://brainly.com/question/28557524
#SPJ9
what chemical element is used as evidence that an asteroid caused the extinction of the dinosaurs?
Answers
The chemical element which is used as evidence that an asteroid caused the extinction of the dinosaurs is Iridium.
Since the 1980s, when scientists discovered asteroid dust in the geologic stratum that marks the extinction of the dinosaurs, experts have held that death by asteroid, rather than a sequence of volcanic eruptions or some other global tragedy, has been the prevailing explanation. This discovery presented a grim image of dust from the destroyed asteroid and boulders from impact orbiting the planet, blotting out the light and causing mass death during a dark, prolonged global winter - all before drifting back to Earth to form the asteroid-enriched layer observable today.
The metal iridium, which is scarce in the Earth's crust but abundant in certain types of asteroids, is a telltale indication of asteroid dust. The asteroid idea was formed from an iridium spike in a geologic strata seen all over the earth.
Learn more about Evidence of extinction :
https://brainly.com/question/7498483
#SPJ4
If 1.00 grams of a compound contains 8.35 x 1021 molecules, what is it molar mass?72.1 g/mol
Answers
The chemical has a molar mass of 72.1 g/mol.
One mole of a chemical has a mass known as its molar mass. The measurement unit is grams per mole (g/mol). We need to know the sample's weight in grams and the number of molecules in order to determine the molar mass of the substance.
In this instance, we are informed that 8.35 x 1021 molecules make up 1.00 grams of the chemical. This knowledge allows us to calculate the molar mass.
We must first determine how many moles are present in the sample. Use the following formula to accomplish this:
Mass of the sample / Molar mass = number of moles
Formula rearranged to account for molar mass:
Molar mass is equal to the mass of the sample divided by the number of moles.
When the values are plugged in, we get:
Sample weight is 1 gram.
8.35 x 1021 molecules are the number of moles.
We can now determine the molar mass:
Molar mass is equal to 1 gram divided by 8.35 x 1021 molecules.
By converting grams to kilograms, we can make the math simpler:
Molar mass is equal to 1.00 x 10-3 kg divided by 8.35 x 1021 molecules.
Now that the units have been cancelled, we can simplify:
Molar mass is calculated as follows: (1.00 x 10-3 kg) / (8.35 x 10-21 molecules) = 1.20 x 10-24 kg/molecule.
The molar mass can finally be converted to grams per mole as follows:
Molar mass is equal to 1.20 x 10-24 kg/molecule times 1000 g/kg times 6.02 x 1023 molecules/mol, or 72.1 g/mol.
To learn more about molar mass click here:
https://brainly.com/question/837939#
#SPJ11
Which statement about the physical change of liquid water boiling into steam is true?
The heat added represents an energy change.
The action cannot be reversed.
O The steam cannot conserve mass.
O The weight lost represents a mass change.
Answers
Answer:
A: The heat added represents an energy change.
Explanation:
Correct on EDG test
which statement regarding the chemical grooming of pyruvate is false? which statement regarding the chemical grooming of pyruvate is false? each pyruvate molecule has a co2 added and then joins with an nadh
Answers
The statement that is false regarding the chemical grooming of pyruvate is "each pyruvate molecule has a CO2 added and then joins with an NADH.
Pyruvate is the end product of glycolysis that further undergoes chemical grooming in the presence of oxygen to produce ATP. The complete oxidation of glucose produces a total of 36-38 ATPs per molecule.
Pyruvate is oxidized to produce Acetyl-CoA. During this process, the carboxyl group of pyruvate is removed and given off as CO2. This is known as decarboxylation.
The remaining 2-carbon molecule is then oxidized by the removal of electrons by the NAD+ which is reduced to NADH. This is called oxidative decarboxylation, and its purpose is to prepare the substrate for energy production.
The correct statement regarding the chemical grooming of pyruvate is, "Each pyruvate molecule loses a CO2 molecule and then joins with a coenzyme A to form acetyl-CoA, producing an NADH molecule."
Learn more about the chemical:
https://brainly.com/question/11231920
#SPJ11
What type of fuel did you read about?
Answers
There are several types of fuels used for various purposes, such as transportation, electricity generation, heating, and cooking.
Fossil Fuels: These are derived from the remains of ancient plants and animals. The three main types of fossil fuels are coal, petroleum (oil), and natural gas. Fossil fuels are the most widely used sources of energy globally.
Renewable Fuels: These are derived from renewable resources and are considered more environmentally friendly. Examples include biofuels (such as ethanol and biodiesel), solar energy, wind energy, and hydropower.
Nuclear Fuel: This refers to the fuel used in nuclear power plants, primarily uranium or plutonium. Nuclear fission is employed to generate heat, which is then converted into electricity.
Hydrogen: Hydrogen can be used as a fuel in various applications, including fuel cells, which produce electricity through a chemical reaction between hydrogen and oxygen.
Alternative Fuels: This category includes unconventional or emerging fuels that aim to reduce greenhouse gas emissions.
For more such question on fuels visit:
https://brainly.com/question/30368384
#SPJ8
Describe the key features of the Sun.
Answers
The Sun is composed of about 75% hydrogen, 25% helium, and trace amounts of heavier elements.
What are the key features of the Sun?Our Sun is a star and it is the near star to planet Earth. The Sun is also the largest thing in our solar system and it contains most of the mass in the whole solar system. Because the Sun has the greatest mass it also has a substantial force of gravity in the solar system.
Without the Sun's heat and light, the Earth would be a lifeless ball of ice-cover rock. The Sun warms our seas, stirs our atmosphere, causes our weather patterns,
So we can conclude that At its core, the Sun's temperature extends over 15 million K (27 million°F) and its pressure is over 200 billion times the pressure at Earth's surface.
Learn more about Sun here: https://brainly.com/question/15837114
#SPJ1
Step 2: measure the area of the top of the syringe
Answers
I am unsure if this is correct, but this might be the whole section:
The top of the syringe is a circle. You need to compute its area for use in later computations of pressure values. Start by using a ruler to measure the diameter. Estimate to the nearest 0.01 cm. Answer: 3.60 cmDivide by two to find the radius. Maintain significant figures. Answer: 1.80 cmSubstitute the radius into the formula A = πr² to find the area of the top of the syringe. Maintain significant figures. Answer: 10.2 cm²The specific heat capacity of water is 1.00 cal/g °C. 700.00 cal is required to raise the temperature of 25.0g water from 22.0°C to 50°C.
What is the final temperature of the above water sample if 1.00kcal of heat is provided?
Answers
When 1.00 kcal of heat is applied, the water sample's final temperature is T = 50.0°C + 40.0°C = 90.0°C.
What does "specific heat" mean?The amount of energy required to raise a substance's temperature is measured in terms of specific heat. It is the amount of energy (measured in joules) required to increase a substance's temperature by one degree Celsius per gram.
We must first determine the water sample's original temperature. The formula is as follows:
Q = mcΔT
Inputting the values provided yields:
700.00 cal = 25.0 g x 1.00 cal/g °C x (50°C - 22.0°C)
When we simplify this equation, we obtain:
ΔT = 700.00 cal / (25.0 g x 1.00 cal/g °C) = 28.0°C
Therefore, the initial temperature of the water sample is 22.0°C + 28.0°C = 50.0°C.
Inputting the values provided yields:
1.00 kcal = 25.0 g x 1.00 cal/g °C x (T - 50.0°C)
When we simplify this equation, we obtain:
T - 50.0°C = 1.00 kcal / (25.0 g x 1.00 cal/g °C) = 40.0°C
Therefore, When 1.00 kcal of heat is applied, the water sample's final temperature is T = 50.0°C + 40.0°C = 90.0°C.
To know more about specific heat visit:-
https://brainly.com/question/11297584
#SPJ1
the movement of positively charged sodium ions across the membrane of a neuron can produce a(n)
Answers
The movement of positively charged sodium ions across the membrane of a neuron can produce an action potential. An action potential is an electrical impulse that travels down the length of the neuron, allowing for communication between neurons.
When a neuron is at rest, there is a higher concentration of sodium ions outside of the cell and a higher concentration of potassium ions inside of the cell. However, when the neuron receives a signal, channels on the cell membrane open, allowing for the influx of sodium ions.
This sudden increase in positive charge triggers the neuron to fire an action potential, which travels down the length of the neuron. Once the impulse reaches the end of the neuron, it triggers the release of neurotransmitters, which carry the signal to the next neuron in the circuit.
Overall, the movement of positively charged sodium ions plays a crucial role in the communication between neurons and the functioning of the nervous system.
you know more about sodium ions across pls visit-
ttps://brainly.com/question/29704319
#SPJ11
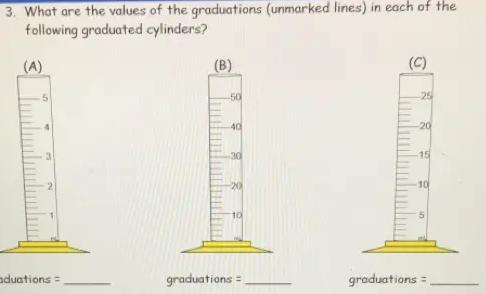
3. What are the values of the graduations (unmarked lines) in each of the
following graduated cylinders?
Q
(A)
50
3
graduations =
(B)
50
-40
-30
-20
10
graduations =
(C)
25
-20
-15
10
5
graduations =
Answers
The values of the Graduations for A , B and C will be 0.2 ml, 2ml and 1ml respectively.
Graduated Cylinder - A popular piece of scientific equipment used to measure the volume of a liquid is a graduated cylinder, sometimes referred to as a measuring cylinder or mixing cylinder. Its form is slender and cylindrical. Each marked line on the graduated cylinder indicates the volume of liquid that has been measured.
The clear graduated cylinder is widely used for volume measurements and is regarded to be more accurate than a beaker since it contains permanently marked incremental graduations.
To calculate the value of graduations-
1. Subtract the two values to get the value between the labeled graduations.
2. Determine how many spaces there are between the two graduations. Keep in mind that volume equals space.
3. Divide the number of gaps by the value between the graduations.
For cylinder A.
2 ml- 1ml = 1ml
Number of spaces = 5
∴1/ 5 = 0.2
Graduation = 0.2ml
For cylinder B -
20-10 = 10
number of spaces = 5
∴10/ 5 =2
Graduation = 2ml
For cylinder C-
10-5 = 5
Number of spaces= 5
∴5/5 = 1
Graduation = 1ml
Hence , Graduations for A , B and C will be 0.2 ml, 2ml and 1ml respectively.
To learn more about graduated cylinders refer-https://brainly.com/question/26216613
#SPJ9
Your question is incomplete. Please find the missing image below.
which of the following statements correctly reflect how to calculate the oxidation number of a covalently bonded atom using electronegativity? select all that apply.
Answers
After assigning electrons based on the Electronegativity value, determine how many (if any) lone pair electrons are on that atom, and add two values together.
The electronegativity of an element increases with oxidation state of the element.
oxidation numbers for covalent bonds:
Although covalent bonds donot result in charges, oxidation states are still useful.
They label hypothetical transfer of electrons if substance were ionic.
Determining oxidation states of atoms in a covalent molecule is very important when analyzing "redox" reactions
To determine Oxidation Number for a non-metal in covalent compounds or polyatomic is the difference between the Group Number and total number of electrons assigned to that atom.
learn more about electronegativity at
https://brainly.com/question/24977425
#SPJ4
the mass of solution is equivalent to the mass of the?
A. solute + solvent
B.solvent
C.solute-solvent
D.solute
Answers
The mass of solution is equivalent to the mass of the solute and solvent.
What is solute?The material whose dissolves is known as a solute, as well as the substance
What is solvent?The solute is dissolved to produce a solution, is known as a solvent.
Solution is made by solvent , solute also. So, by adding mass of solvent and solute mass of a particular solution could be found.
It can be expressed as:
Mass of solution = mass of solute + mass of solvent
Therefore, the mass of solution is equivalent to the mass of the solute and solvent.
To know more about solute and solvent.
https://brainly.com/question/14797683
#SPJ2