Answers
Answer:
Explanation:
M1V1=M2v2
M1=0.150M
M2=0.150M
V2=120*10^-3
V1=M2V2/M1
V1=0.150*120*10^-3/0.150
V1=0.12
0.12 mL volume of 0.150M 2M Li₂S solution is required to completely react with 120 mL of 0.150M Co(NO₃)₂ MCo(NO₃)₂.
What is the relation of volume with molarity?
With M1 and M2 representing the molarity of the solutions, expressed as mol/L or M, and V1 and V2 representing their respective volumes, this calculator may be used to calculate a missing number for the dilution equation.
A solution's concentration is determined by multiplying its volume by its molarity (M1V1 = M2V2) On both sides of the equation, the units should stay the same.
Here
M₁V₁=M₂V₂
M₁=0.150M
M₂=0.150M
V₂=120 × 10⁻³
V₁=M₂V₂/M₁
V₁=0.150 × 120 × 10⁻³/0.150
V₁=0.12.
Therefore, 0.12 mL volume of 0.150M 2M Li₂S solution is required to completely react with 120 mL of 0.150M Co(NO₃)₂ MCo(NO₃)₂.
Read more about volume and molarity, here
https://brainly.com/question/4172228
#SPJ2
Related Questions
A chemist combusts a 1.87g sample of octane (C_{R}*H_{18}) completely in a lab. The chemist does not pass out after the combustion is complete . The balanced equation for the combustion is: 2C 8 H 18 00 +25O 2(0) 16CO 2(0) +18H 2 O (00) What is the limiting reactant in this reaction ? Answer in two or more sentences , cite evidence and justify your claim .
Answers
The amount of oxygen needed in the reaction to burn the octane is higher than necessary. Octane is hence the reaction's limiting agent.
In chemistry, what exactly is a limiting reagent?The limiting reactant, also known as the limiting reagent, is the reactant that is used up first during a chemical reaction, hence restricting the amount of product that can be produced during the course of the reaction.
What are excess and limiting reagent, respectively?A chemical reaction's limiting reagent is the one that will totally consume all of the reactants. The reaction can't continue once there is no more of a reactant. Hence, it prevents the reaction from intensifying and continuing further. If the other reactant hadn't been used up completely, the surplus reagent would have continued to react, and the reaction would also have continued.
To learn more about limiting reagent visit:
brainly.com/question/11848702
#SPJ1
HQ5.40
Homework Answered Due Today, 11:59 PM
The reaction 3H₂(g) + N₂(g) → 2NH3(g) has an enthalpy of reaction of -92.6 kJ/mol. If 1 g of hydrogen and 2 g of nitrogen are
reacted, how much heat is produced (kJ)?
Answers
The amount of heat energy produced when 1 g of hydrogen and 2 g of nitrogen are reacted, is -6.61 KJ
How do i determine the heat energy produced?First, we shall obtain the limiting reactant. Details below:
3H₂ + N₂ -> 2NH₃
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 g Molar mass of H₂ = 2 g/molMass of H₂ from the balanced equation = 3 × 2 = 6 gFrom the balanced equation above,
28 g of N₂ reacted with 6 g of H₂
Therefore,
2 g of N₂ will react with = (2 × 6) / 28 = 0.43 g of H₂
We can see that only 0.43 g of H₂ is needed in the reaction.
Thus, the limiting reactant is N₂
Finally, we the amount of heat energy produced. Details below:
3H₂ + N₂ -> 2NH₃ ΔH = -92.6 KJ
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 gFrom the balanced equation above,
When 28 grams of N₂ reacted, -92.6 KJ of heat energy were produced.
Therefore,
When 2 grams of N₂ will react to produce = (2 × -92.6) / 28 = -6.61 KJ
Thus the heat energy produced from the reaction is -6.61 KJ
Learn more about heat energy:
https://brainly.com/question/31429264
#SPJ1
A buffered solution _______. Select the correct answer below: fails to keep hydronium and hydroxide ion concentrations nearly constant when strong acids or bases are added. maintains a constant or nearly constant pH when small amounts of strong acids or bases are added. acts to keep the hydroxide ion concentration nearly constant. acts to keep the hydronium ion concentration nearly constant.
Answers
The correct option for the given question about Buffer Solution is Option B) which is maintains a constant or nearly constant pH when small amounts of strong acids or bases are added.
What is a Buffer Solution?When a little quantity of acid or base is diluted or added, the buffer solution undergoes very slight variations in its hydrogen ion concentration (pH). pH may be maintained in buffer solutions, which are mixtures of a weak acid and its conjugate base or a weak base and its conjugate acid.Acidic and alkaline buffers are the two main groups into which buffer solutions are commonly categorized.A weak acid and its salt are combined with a strong base to create an acidic buffer, which has an acidic pH.A weak base, its salt, and a strong acid are combined to create an alkaline buffer, which has a basic pH.
Thus we conclude that when weak acids or bases are supplied in small amounts, the pH remains steady or almost constant.
Learn more about Buffer Solution here:
https://brainly.com/question/26416276
#SPJ2
You hear about a new amazing fertilizer called SuperGro. The company selling it claims that using this fertilizer will double your tomato harvest! Sounds great but as a skeptical consumer you ask for a sample so that you can conduct an experiment in your garden before you buy a big bag of his fertilizer.
state a good hypothesis for your fertilizer experiment describe the experiment you would conduct .identify the control and experimental groups/treatments . identify the dependent and independent variables
• name two control variables
•make up hypothetical results
• state a conclusion
Answers
Answer:
Hypothesis: If I use SuperGro fertilizer on my tomato plants, then the harvest will be greater than if I do not use the fertilizer.
Experiment: I would divide my tomato plants into two groups. The first group would be the control group and receive no fertilizer. The second group would be the experimental group and receive SuperGro fertilizer. I would use the same type and number of tomato plants in both groups and plant them in the same location with the same amount of sunlight and water.
Control group: Tomato plants that receive no fertilizer
Experimental group: Tomato plants that receive SuperGro fertilizer
Independent variable: SuperGro fertilizer
Dependent variable: Tomato harvest
Control variables: type and number of tomato plants, location, amount of sunlight and water
Hypothetical results: The tomato plants in the experimental group that received SuperGro fertilizer produced double the amount of tomatoes compared to the tomato plants in the control group that did not receive fertilizer.
Conclusion: Based on the results of the experiment, it can be concluded that SuperGro fertilizer does have a significant effect on increasing tomato harvest. Therefore, using SuperGro fertilizer can be recommended for individuals who want to increase their tomato harvest.
Explanation:
Draw and label a picture of an ozone (O3) molecule (Hint start with an O2 then attach the third O). What type of bond is used to attach the 3rd oxygen atom to the ozone molecule? Explain in words how this bond forms.
Answers
The ozone molecule is composed of three atoms of the oxygen.
What is the structure of the ozone molecule?The ozone molecule (O3) is a triatomic molecule, meaning that it consists of three atoms. It is composed of three oxygen atoms, which are held together by covalent bonds.
The structure of the ozone molecule can be described as a bent or V-shaped molecule, with the three oxygen atoms arranged in a triangular fashion. The central oxygen atom is bonded to two other oxygen atoms, which are located above and below it, with bond angles of approximately 117 degrees.
Learn more about Ozone:https://brainly.com/question/14330630
#SPJ1

why do you think danny taught that way
Answers
Answer:
I wonder why...
Explanation:
If you drive a car to work, what fraction of the chemical energy of the gasoline ultimately ends up as heat?
Answers
Answer:
All of the chemical energy of the gasoline ends up as heat
Explanation:
Gasoline has chemical energy stored in it. When used as a fuel to power a car, the gasoline is combusted to convert the chemical energy stored within into heat energy, and power the car. The heat energy is further converted to mechanical energy, light energy, sound energy, etc.
How many moles are in 74.8 grams of NaCl?
Answers
Answer:
1.28 mol NaCl
Explanation:
Find the mass of NaCl by adding the two elements atomic mass
Na: 22.99
Cl: 35.45
22.99+35.45=58.44 g
Now convert to moles
74.8 g / 58.44 g = 1.27994524298 mol
Its DUE TODAY AT 6:00PM!!!!!!!!
Would you expect the reaction of phosphoenolpyruvate to pyruvate to be a reversible reaction?
Answers
Answer:
i) In thee mitochondrion, pyruvate carboxylase converts pyruvate to oxloacetate.
ii) Malate dehydrogenase in the mitochondrion reduces oxaloacete to to malate.
iii) Malate dehydrogenase in the cytoplasm oxidizes malate to oxaloacetate.
iv) Phosphoenolpyruvate carboxykinase decarboxylates and phosphorylates oxaloacetate.forming phosphoenolpyruvate.
Explanation:
Gluconeogenesis is the synthesis of glucose from non - carbohydrate compounds. The substrates for gluconeogenesis are lactate, pyruvate, amino acids, propionate and glycerol.
Gluconeogenesis occurs only in cytosol but the precursor is produced in mitochondria. In the conversion of pyruvte to phosphoenolpyruvate occur in mitochondria and cytosol.
Step -1:
Pyruvate carboxylase is a biotin dependent enzyme located in mitochondria. It converts pyruvate to oxlaoacetate and carbondioxide in the presence of ATP.Oxlaocetate synthesized in mitochondrial matrix has to be transported to cytosol for gluconeogenesis. Oxaloacetate is impermeble, cannot be sent out of mitochondria. So it has to be converted to malate.
Step -2:
Malate dehydrogenase in mitochondria converts oxaloacetate synthesized in mitochondrial matrix to malate. And then it is transported to cytosol.
Step 3:
Malate dehydrogenase responsible for reversible reaction in cytosol converts malate to oxaloacetate.
Step -4
Phosphoenolpyruvate carboxy-kinase in cytosol converts oxaloacetate to PEP. The enzyme transfer high energy phosphate bond from GTP to oxaloacetate to from PEP and liberated carbondioxide.
Therefore, the steps of glycolysis converts phosphoenolpyruvate to pyruvate are as follows.
i) In thee mitochondrion, pyruvate carboxylase converts pyruvate to oxloacetate.
ii) Malate dehydrogenase in the mitochondrion reduces oxaloacete to to malate.
iii) Malate dehydrogenase in the cytoplasm oxidizes malate to oxaloacetate.
iv) Phosphoenolpyruvate carboxykinase decarboxylates and phosphorylates oxaloacetate.forming phosphoenolpyruvate.
Explanation:
If you have six molecules of CO2how many oxygen atoms do you have?
Answers
When our calculator math provides the value 0.0021471, but we need to record the value with only three significant figures, what would we record?
Answers
The number that is given as digits is established using significant figures. A meaningful representation of numbers is carried by these digits. Frequently, significant digits are employed in place of figures.
Thus, By counting all of the values beginning with the first non-zero digit on the left, we may determine the number of significant digits.
The crucial or important digits that accurately represent the meaning of a certain number are known as the significant figures of that number.
6.658, for instance, has four significant digits. These huge amounts give the numbers accuracy. Additionally, they are known as significant digits.
Thus, The number that is given as digits is established using significant figures. A meaningful representation of numbers is carried by these digits. Frequently, significant digits are employed in place of figures.
Learn more about Significant figures, refer to the link:
https://brainly.com/question/23396760
#SPJ1
In an ionic compound, the size of the ions affects the internuclear distance (the distance between the centers of adjacent ions), which affects lattice energy (a measure of the attractive force holding those ions together). Based on ion sizes, arrange these compounds by their expected lattice energy. Note that many sources define lattice energies as negative values. Please arrange by magnitude and ignore the sign.
Compunds: RbCl ,RbBr ,Rbl ,RbF
Answers
Answer:
The correct answer will be " RbF > RbCl > RbBr > Rbl".
Explanation:
The size of the given ions will be:
RbCl:
⇒ 689kJ/mol
RbBr:
⇒ 660kJ/mol
Rbl:
⇒ 630kJ/mol
RbF:
⇒ 785kJ/mol
Now according to the size, the arrangement will be:
⇒ (785kJ/mol) > (689kJ/mol) > (660kJ/mol) >(630kJ/mol)
⇒ RbF > RbCl > RbBr > Rbl
The bond among all opposite charging ions seems to be strongest whenever the ions were indeed small.
When 3,3-dimethylbut-1-ene is treated with HBr alone, the major product is 2-bromo-2,3-dimethylbutane. When the same alkene is treated with HBr and peroxide, the sole product is 1-bromo-3,3-dimethylbutane. Explain these results by referring to the mechanisms.
Answers
It yields 2-bromo-2,3-dimethylbutane as the final product. The proton is added to the radical process after the bromine anion. 1-bromo-3,3-dimethylbutane.
Which of the following types of chemicals results from an alkene's hydration?1 Response. Alcohol is created when alkene and water interact. Electrophilic addition is the reaction at hand. The double bond of the carbon in an alkene with a high electron density is attacked by water.
What do examples of hydration of alkenes mean?Alkene Hydration Example: ethanol is produced by the hydration of ethylene. An alkene with the condensed structural formula H2C=CH2, ethylene is also known as. The double bond (C=C) in the ethene molecule is the active site.
to know more about dimethylbutane here:
brainly.com/question/16942355
#SPJ4
for the follwing pair of polymers,do the following:(1)state whether it is possible to decide if one polymer has a higher tensile modulus than the other;(2)if this is possible,note which has the higher tensile modulus and then cite the reason(s) for your choice;and (3) if it is not possible to decide,then state why not.
-syndiotactic polystyrene having a number-average molecular weight of 400,000g/mol
-isotactic polystyrene having a number-average molecular weight of 650,000g/mol
8.8 for the follwing pair of polymers,do the following:(1)state whether it is possible to decide if one polymer has a higher tensile modulus than the other;(2)if this is possible,note which has the higher tensile modulus and then cite the reason(s) for your choice;and (3) if it is not possible to decide,then state why not.
-syndiotactic polystyrene having a number-average molecular weight of 600,000g/mol
-isotactic polystyrene having a number-average molecular weight of 500,000g/mol
Answers
Yes, it is possible.
The tensile modulus of the linear and isotactic poly(vinyl chloride) will be higher. Compared to branched polymers, linear polymers are more prone to crystallize.
What is the mechanical properties of polymers?
The moduli of elasticity and other classes of strength measures, such as the yield and tensile strengths, can be used to characterize the mechanical properties of polymers in a manner that is quite similar to that of metals or other common crystalline materials. The most significant mechanical and elastic characteristics of polymeric materials are summarized generally below:
Strength: Whenever the applied force is greater than a straightforward linear elastic deformation, represents the stress force required to fracture the material sample under investigation. Tensile strength (stretching of the polymer), compressional strength (compressing the polymer), flexural strength (bending of the polymer), torsional strength (twisting of the polymer), and impact strength are some of the most important types of strength quantities used in typical materials characterization measurements (e.g. under the effects of direct hammering).Percent Elongation to Break (Ultimate Elongation): This statistic, expressed as a percentage change in the material's length, describes the maximum strain that the polymer sample can experience before it ultimately breaks (at the aforementioned strength point).Young's Modulus: A measure of a material's total stiffness, it is the ratio of stress to strain in the region of its linearly elastic response.To know more about Mechanical properties of polymers, visit:
https://brainly.com/question/28271394
#SPJ4
Which portion of a molecule of F2O has partial positive charge?
Question 3 options:
A)
The F atoms
B)
The central O atom
C)
The partial charge on each atom is zero
D)
The partial charge on each atom is negative
Answers
The partial charges on each fluorine atom are negative. Option B) The central O atom is the correct answer. Option B
The partial charges in a molecule are determined by the electronegativity values of the atoms involved. Electronegativity is the ability of an atom to attract electrons towards itself in a chemical bond. In the case of \(F_2O\), fluorine (F) is more electronegative than oxygen.
Fluorine is the most electronegative element on the periodic table, meaning it has a high ability to attract electrons. Oxygen is also relatively electronegative but less so than fluorine. When fluorine atoms bond with oxygen, the shared electrons will be pulled more towards the fluorine atoms, creating a polar covalent bond.
In \(F_2O\), each fluorine atom will pull the shared electrons towards itself, resulting in a higher electron density around the fluorine atoms. This creates a region of partial negative charge around the fluorine atoms.
Conversely, the oxygen atom will have a region of lower electron density and, therefore, a partial positive charge. This is because the shared electrons spend more time around the fluorine atoms due to their higher electronegativity.
Option B
For more such question on partial charges visit:
https://brainly.com/question/29974793
#SPJ8
Which relationship or statement best describes ΔS° for the following reaction?
KCl(s) → K+(aq) + Cl−(aq)
Explain why.
A. ΔS° ≈ 0
B. ΔS° = ΔH°/T
C. ΔS° > 0
D. ΔS° < 0
E. More information is needed to make a reasonable prediction.
Answers
The ΔS° value for the reaction KCl(s) → K+(aq) + Cl−(aq) is ΔS° > 0, as the products have a higher degree of disorder than the reactant due to an increase in the number of particles in solution. Hence the correct option is (C) ΔS° > 0.
The ΔS° value for a reaction represents the change in the entropy of the system, which is a measure of the disorder or randomness of the system. The reaction KCl(s) → K+(aq) + Cl−(aq) involves a solid compound breaking down into two separate aqueous ions, which means that the products have a higher degree of disorder than the reactant. This increase in the number of particles in solution results in an increase in entropy, which means that ΔS° > 0. Option (A) is incorrect because the reaction involves a change in state, which results in an increase in entropy. Option (B) is incorrect because it represents the relationship between enthalpy and entropy, not the ΔS° value for this particular reaction. Option (D) is incorrect because the reaction results in an increase in entropy, not a decrease. Option (E) is incorrect because the given information is sufficient to predict the sign of ΔS°.
To know more about reaction please refer: https://brainly.com/question/28984750
#SPJ1
calculate the number of moles for the quanity 8.06 x 1021 atoms of Pt
Answers
The number of moles for the quanity 8.06 x\(10_{21\) atoms of Pt is approximately 2.61 grams.
To calculate the number of moles for a given quantity of atoms, we can use Avogadro's number and the molar mass of the element. Avogadro's number is 6.022 x 10²³ atoms/mol.
In this case, you have 8.06 x 10²¹ atoms of Pt. To find the number of moles, divide this quantity by Avogadro's number:
8.06 x 10²¹ atoms Pt / 6.022 x 10²³ atoms/mol = 0.0134 mol Pt
So, there are approximately 0.0134 moles of Pt in 8.06 x 10²¹ atoms of Pt.
The molar mass of Pt (platinum) is 195.08 g/mol. To convert the number of moles to grams, multiply the number of moles by the molar mass:
0.0134 mol Pt x 195.08 g/mol = 2.61 g Pt
Therefore, there are approximately 2.61 grams of Pt in 8.06 x10²¹ atoms of Pt.
In summary, the number of moles for the quantity 8.06 x 10²¹ atoms of Pt is approximately 0.0134 moles. This is equivalent to approximately 2.61 grams of Pt. Remember to use Avogadro's number and the molar mass to perform these calculations accurately.
Know more about moles here:
https://brainly.com/question/29367909
#SPJ8
How many full orbitals are in phosphorus
Answers
Answer:
three half-filled orbitals
Answer:
6p
Explanation:
It can hold a total of 6
What is the mass, in grams, of 5.90 mol C8H18?
Answers
\(\huge\mathbb{✒ANSWER}\)
HI BRAINLY USER!!
JUST CLICK THE ATTACHMENT PHOTO⊱─━━━━━━━━━⊱༻●
༺⊰━━━━━━━━━─⊰
》Mass = 5.90 x 114 = 672.6g.This can be rounded to 673g
⊱─━━━━━━━━━⊱༻
●༺⊰━━━━━━━━━─⊰
#CARRY ON LEARNING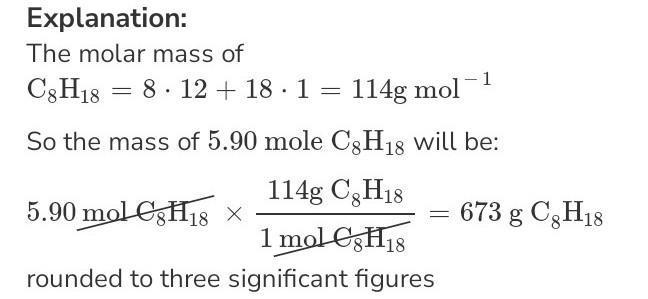
As you move down a group the electronegativity of an element will
Answers
orbital designation of n=4 l=0 ml=0
Answers
Cr is the element with the such orbital designation.
What is the purpose of the metal chromium?Chromium is used to harden steel, to make stainless steel (so-called because it does not rust), and to make a variety of compounds. Steel can be given a polished mirror surface with chromium plating. Bumpers and other car and truck parts plated in chromium were once very prevalent.
What is Cr's atom configuration?Because chromium has two electrons in its 3s and 4s subshells, it will have a 3d⁵ 4s¹ electron configuration to occupy those two subshells. The 3d⁵ subshell can be filled with two electrons, which is the most probable configuration. Chromium's valence electrons are all in distinct orbitals.
learn more about chromium here
https://brainly.com/question/28614686
#SPJ9
the question is incomplete. the complete question is
The orbital designation of n=4 l=0 ml=0 s=+1/2 corresponds to which of the following atoms?
Na
Cl
Cr
Br
Sodium (Na) and chlorine (CI) combine to form a __ of salt (NaCI)
A. Mixture
B. Molecule
Answers
Answer:A mixture
Have a nice day :D
all atoms of the same elements have the same what?
Answers
Answer:
The same number of proteins in their nucleus.
Answer:
Below
Explanation:
All atoms of the same element have the same amount of protons! This is because the number of protons that exist in the nucleus is the element's atomic number.
Hope this helps!
An objects weighs 88.02 oz. What is the mass for this object in g?
Answers
Answer:
2464.56 grams
Explanation:
To convert ounces to grams, you multiply the given ounces by 28.
88.02 x 28 = 2462.56 grams
Hope this helped! :^)
The weight of object is 2495.37 in g.
What is mass ?Mass is a measure of the amount of matter that an object contains.
The base SI unit of mass is the kilogram or kg
It was originally defined as the mass of 1L of liquid water at 4oC (the volume of a liquid changes slightly with temperature).
1 ounce = 28.35 grams
The given weight is 88.02 oz
Therefore in grams it will be
= 88.02*28.35
= 2495.37 g
Therefore the mass of object is 2495.37 in g.
To know more about mass
https://brainly.com/question/19694949
#SPJ2
Volcanic eruptions inflation

Answers
The volcanic eruptions inflation are listed below.
What is volcanic eruption ?
Lava and gas are occasionally discharged explosively from a volcano during a volcanic eruption. When newly erupted lava cascades down a volcano's flanks, it is known as a "glowing avalanche" and is the most deadly sort of eruption. Temperatures of up to 1,200 degrees Fahrenheit can be reached and they can move swiftly.
What is eruption?
The silica content and gas content of the magma play a major role in determining the features of the four different types of eruptions. These include the Hawaiian, Strombolian, Vulcanian, and Plinian eruptions, in order of increasing explosiveness.
The earth surface often expands when magma builds up in an underground reservoir prior to an eruption (named inflation). Similarly, as magma exits the reservoir with the potential to erupt, the land above the reservoir sinks (named deflation).
Therefore, volcanic eruptions inflation are listed above.
Learn more about volcanic eruption from the given link.
https://brainly.com/question/25121802
#SPJ1
If during the investigation it is found that mineral A can scratch Mineral B, but Mineral B cannot scratch Mineral A, then which of these statements is true?
Mineral A is smoother than Mineral B
Mineral A is rougher than Mineral B
Mineral A is harder than Mineral B
Mineral A is softer than Mineral B
Answers
Answer:
Mineral A is harder than mineral B
Which of these is not a safety decision?
O A. Using a material that will fail in a safe way
OB. Using a material that is sustainable and environmentally friendly
O C. Choosing a material that will show warning before failing
D. Choosing a material that will fail in a predictable way

Answers
Answer:
B is the answer
Explanation:
Answer:the answer was B , he is correcf
Explanation:
How many isomers does propane have?
01
02
03
05
Answers
Answer:
A.) 1
Explanation:
Propane only exists in one conformation. It does not have enough carbons to form branches, and there are only hydrogens attached to each carbon. Furthermore, there is no way to twist the carbon or change its orientation (ex. cis- and trans-) to result in a different structure of propane. There is no other way to represent the molecule without drawing a different molecule.
Given that 4 NH3 + 5 O2 → 4 NO + 6 H2O, if 3.00 mol NH3 were made to react with excess of oxygen gas, the amount of H2O formed would be
Answers
x mol H2O = 4.50 mol H2O
Step-by-step explanation:
From the balanced equation, we can see that for every 4 moles of NH3 that react, 6 moles of H2O are formed. Therefore, we can use a proportion to find the amount of H2O that would be formed if 3.00 mol of NH3 reacted:
4 mol NH3 : 6 mol H2O = 3.00 mol NH3 : x mol H2O
Solving for x, we get:
x mol H2O = (6 mol H2O / 4 mol NH3) * 3.00 mol NH3
x mol H2O = 4.50 mol H2O
Therefore, if 3.00 mol of NH3 were made to react with excess oxygen gas, 4.50 mol of H2O would be formed.
2. If I have an unknown quantity of gas at a pressure of 1.22 atm, a volume of31.5L, and a temperature of 89.5°C, how many moles of gas do I have?
Answers
Answer
n = 1.29 moles
Explanation
Given:
Pressure (P) = 1.22 atm
Volume (V) = 31.5 L
Temperature =89.5 C = 362.5 K
We know R = 0.082 L.atm/K.mol
Required: moles of the unknown gas
Solution:
Use the ideal gas law:
PV = nRT
n = PV/RT
n = (1.22 atm x 31.5 L)/(0.082 L.atm/K.mol x 362.5 K)
n = 1.29 moles