What type of compound is HCI?
A. Ionic
B. Molecular
C. Acid
D. Hydrate
Answers
Related Questions
Gentamycin crystals are filtered though a small test.a. Trueb. False
Answers
The statement "Gentamycin crystals are filtered through a small test" is unclear and lacks sufficient context to provide a definitive answer.
However, I can provide some general information about gentamicin and filtration.
Gentamicin is an antibiotic commonly used to treat bacterial infections. It is available in various forms, including solutions for injection and topical application.
Filtration is a process used to separate particles or impurities from a solution or suspension. It involves passing the solution through a filter, which retains the particles and allows the clear liquid to pass through.
If the intent of the statement is to say that gentamicin crystals are filtered through a small filter as part of the manufacturing process, this could be possible.
Gentamicin is typically produced as a powder, and filtering the crystals through a small filter could help remove any impurities and ensure a consistent particle size.
However, without additional context, it is impossible to say for certain whether gentamicin crystals are filtered through a small test.
It is also worth noting that the process of manufacturing pharmaceuticals involves many steps, and filtration is just one of them. Other steps may include purification, drying, and milling, among others.
To know more about Gentamycin crystals refer here
https://brainly.com/question/28538104#
#SPJ11
rank the nitrogen‑containing aromatic molecules in order of increasing basicity. you are currently in a ranking module. turn off browse mode or quick nav, tab to move, space or enter to pick up, tab to move items between bins, arrow keys to change the order of items, space or enter to drop. least basic most basic
Answers
The order of increasing basicity is: Aniline < Pyridine < Pyrrole < Ammonia.
To rank the nitrogen-containing aromatic molecules in order of increasing basicity, we need to consider the electron-donating ability of each molecule's nitrogen atom. The more electron-donating the nitrogen atom, the more basic the molecule.
1. Aniline (\(C_6H_5NH_2\)): Aniline is the least basic molecule among the given options. The nitrogen atom in aniline is directly attached to an aromatic ring, which has a partial negative charge. This partial negative charge reduces the electron-donating ability of the nitrogen atom, making it less basic.
2. Pyridine (\(C_5H_5N\)): Pyridine is more basic than aniline. The nitrogen atom in pyridine is also attached to an aromatic ring, but the nitrogen atom in pyridine is less affected by the partial negative charge of the ring. As a result, the nitrogen atom in pyridine can donate electrons more easily, making it more basic than aniline.
3. Pyrrole (\(C_4H_5N\)): Pyrrole is more basic than pyridine. The nitrogen atom in pyrrole is directly involved in a conjugated pi-system, which provides additional electron density to the nitrogen atom. This increased electron density allows the nitrogen atom in pyrrole to donate electrons more readily, making it more basic than pyridine.
4. Ammonia (\(NH_3\)): Ammonia is the most basic molecule among the given options. Unlike the previous three molecules, ammonia is not aromatic. However, it is still a nitrogen-containing compound. The lone pair of electrons on the nitrogen atom in ammonia is not involved in any aromatic or conjugated pi-system, making it highly available for donation. This makes ammonia the most basic among the given molecules.
For more such question on basicity visit:
https://brainly.com/question/172153
#SPJ8
PLEASE HELP! I will give brainliest
What mass of NaCl is present in 50.0 mL of 1.05 M NaCl solution?
Answers
Answer:
3.07g of NaCl are present in the solution
Explanation:
A 1.05 M NaCl solution means you have 1.05 moles of NaCl per liter of solution. In 50.0mL = 0.0500L you will have:
0.0500L * (1.05mol / L) = 0.0525 moles of NaCl.
To convert these moles to grams you must use molar mass of the compound (Molar mass NaCl: 58.44g/mol). In 0.0525 moles of NaCl you will have:
0.0525mol * (58.44g / mol) =
3.07g of NaCl are present in the solutionWhat ma of potaium chlorate i needed to generate 114. 0 L of O2 at 0. 720 atm and 291 K?
Answers
The mass of potassium chlorate is 0.3439 to generate 114.0 L \(O_{2}\) at 0.720 atm and 291K
The decomposition of potassium chlorate is represented as
\(2KClO_{3} (s) - > 2KCl(s) + 3O_{2} (g)\)
Given, Volume = 114.0 L
pressure = 0.720 atm
temperature = 291 K
According to the Ideal gas Equation
Pv = nRT
\(n=\frac{Pv}{RT}\)
\(n=\frac{0.720*114.0}{0.82*291}\)
n= 0.3439
To know more about Ideal Gas visit:
https://brainly.com/question/25290815
#SPJ4
In a many-electron atom, only the electrons at the ______ level will participate in covalent bonding.
Answers
In a many-electron atom, only the electrons at the outermost energy level will participate in covalent bonding.
The valence shell is the outermost energy level of an atom. It contains the electrons that are most likely to be involved in chemical interactions, such as covalent bonding. Covalent bonding occurs when two atoms share one or more electrons in order to achieve a more stable electron configuration. The electrons in the valence shell of each atom are the ones that are shared in this process. Therefore, only the electrons in the valence shell of a many-electron atom will participate in covalent bonding.
To learn more about covalent bonding click here https://brainly.com/question/14509196
#SPJ11
Extra points please someone help

Answers
Answer: Plants and animals share many characteristics, but they are different in some respects hope that helps-
Explanation:
the average diameter of an adult human eyeball is 24mm what is the diameter in dm
Answers
Answer: 0.24 dm
Explanation:
Which of the following is an element?
A. Aluminum
B. Carbon
C. Gold
D. All of the above
Answers
give the major product for each of the following reactions 2 pentanol h3po4
Answers
The major product of the given reaction is 2-pentene, which is obtained through an elimination reaction involving the removal of hydrogen from the alcohol molecule to form an alkene molecule. Dehydration reactions are chemical reactions in which two molecules are combined to form one larger molecule, or where a water molecule is removed from a larger molecule to form a smaller molecule.
The major product for the given reaction, which is 2 pentanol with H3PO4, is 2-pentene.The reaction of 2-pentanol with phosphoric acid (H3PO4) undergoes an elimination reaction to give 2-pentene as the major product. The reaction is called dehydrogenation since it involves the removal of hydrogen from the alcohol molecule to form an alkene molecule. A dehydration reaction is a chemical reaction in which two molecules are combined to form one larger molecule while a dehydration reaction involves the removal of a water molecule from a larger molecule to form a smaller molecule.
To know more about Dehydration Visit:
https://brainly.com/question/29262706
#SPJ11
An amide that has a molecular ion with an m/z value of 129.
Express your answer as a molecular formula. Enter the elements in the order: C, H, N, O.
Answers
An amide that has a molecular ion with an m/z value of 129.
The molecular formula is C₉H₁₃NO.
To determine the molecular formula of the amide with an m/z value of 129, we need to consider the possible combinations of carbon (C), hydrogen (H), nitrogen (N), and oxygen (O) that would yield that molecular mass.
The m/z value of 129 indicates the mass-to-charge ratio of the molecular ion. Since we're dealing with a neutral molecule, we can assume a charge of +1 for the molecular ion. Therefore, the molecular mass would be equal to 129.
To find the molecular formula, we can consider different combinations of elements that sum up to a molecular mass of 129. Here are a few possibilities:
1. C₈H₁₁NO: In this case, the sum of the atomic masses is (8 × 12.01) + (11 × 1.01) + 14.01 + 16.00 = 128.09, which is close to the desired molecular mass but not exactly 129.
2. C₈H₁₀N₂O: In this case, the sum of the atomic masses is (8 × 12.01) + (10 × 1.01) + (2 × 14.01) + 16.00 = 128.14, which is also close to 129 but not exact.
3. C₉H₁₃NO: In this case, the sum of the atomic masses is (9 × 12.01) + (13 × 1.01) + 14.01 + 16.00 = 129.12, which is very close to 129.
Therefore, the molecular formula that best fits the given m/z value of 129 is C₉H₁₃NO.
To know more about m/z value here
https://brainly.com/question/31482540
#SPJ4
which gas is not an example of a naturally occurring greenhouse gas?responsesnitrous oxidenitrous oxidemethanemethanechlorofluorocarbonchlorofluorocarbonwater vapor
Answers
Chlorofluorocarbons (CFCs) are not naturally occurring greenhouse gases.
Chlorofluorocarbons (CFCs) are not naturally occurring greenhouse gases. They are synthetic compounds that were widely used in refrigerants, solvents, and aerosol propellants.
CFCs were found to deplete the ozone layer in the upper atmosphere, leading to the adoption of the Montreal Protocol to phase out their use.
While they are not naturally occurring, they contribute significantly to global warming due to their strong greenhouse effect.
To know more about Chlorofluorocarbons refer here:
https://brainly.com/question/16159504
#SPJ11
Determine the percent yield for the reaction between 82.4g of Rb and 11.6g of O2 if 39.7f of Rb2O is produced?
Answers
The reaction has a 44.06% yield as a percentage. 0.5 g of product are produced during a chemical process. The calculated yield is 1.6 g at its highest level.
What does a reaction mean in chemistry?When atoms establish or break chemical bonds, reactions take place. Reactants are the substances that start a chemical reaction, while products are the substances that come out of the process.
What is a reaction, simply put?A response is an action that is made in response toward something. You may tell if your parents are upset if you tell them you would really like to move out by their response. When a chemical interacts with another substance, a chemical reaction occurs.
To know more about Reaction visit:
https://brainly.com/question/28270550
#SPJ1
Please answer all of the examples below!

Answers
Answer:
The first one is balanced. The second one is not.
Explanation:
The first one is balanced because there are the same amount of elements on the reactant side as there are on the product side. The second one is not balanced because there are not the same amount of elements on the reactant side as there are on the product side. Therefore, it is not balanced
More Detailed: first example
Reactant side (left of arrow)
H: 2
C: 1
O: 3
Product side (right of arrow)
H: 2
C: 1
O: 3
More detailed: second example
Reactant side:
Na: 1
O: 1
H: 1
C: 1
Product side:
Na: 2
O: 4
H: 2
C: 1
the variables needed to balance this equation is 2,1, 1, 1.
which transition state is more stable and why? select an answer and submit. for keyboard navigation, use the up/down arrow keys to select an answer. a the transition state that produces the markovnikov product is more stable because a partial positive charge is forming on tertiary carbon your answer b the transition state that produces the markovnikov product is more stable because a partial positive charge is forming on primary carbon c the transition state that produces the anti-markovnikov product is more stable because a partial positive charge is forming on tertiary carbon d the transition state that produces the anti-markovnikov product is more stable because a partial positive charge is forming on primary carbon
Answers
Answer:
The closer the transition state to the product, the more stable it is. It is because this transition state is closest to the final form of the product, which is usually stable
What causes solid to change to a liquid?
Answers
Answer:
heat
Explanation:
it causes the atoms to move faster and the bonds between them become looser, and the solid changes to a liquid.
can anybody plz hep me on this



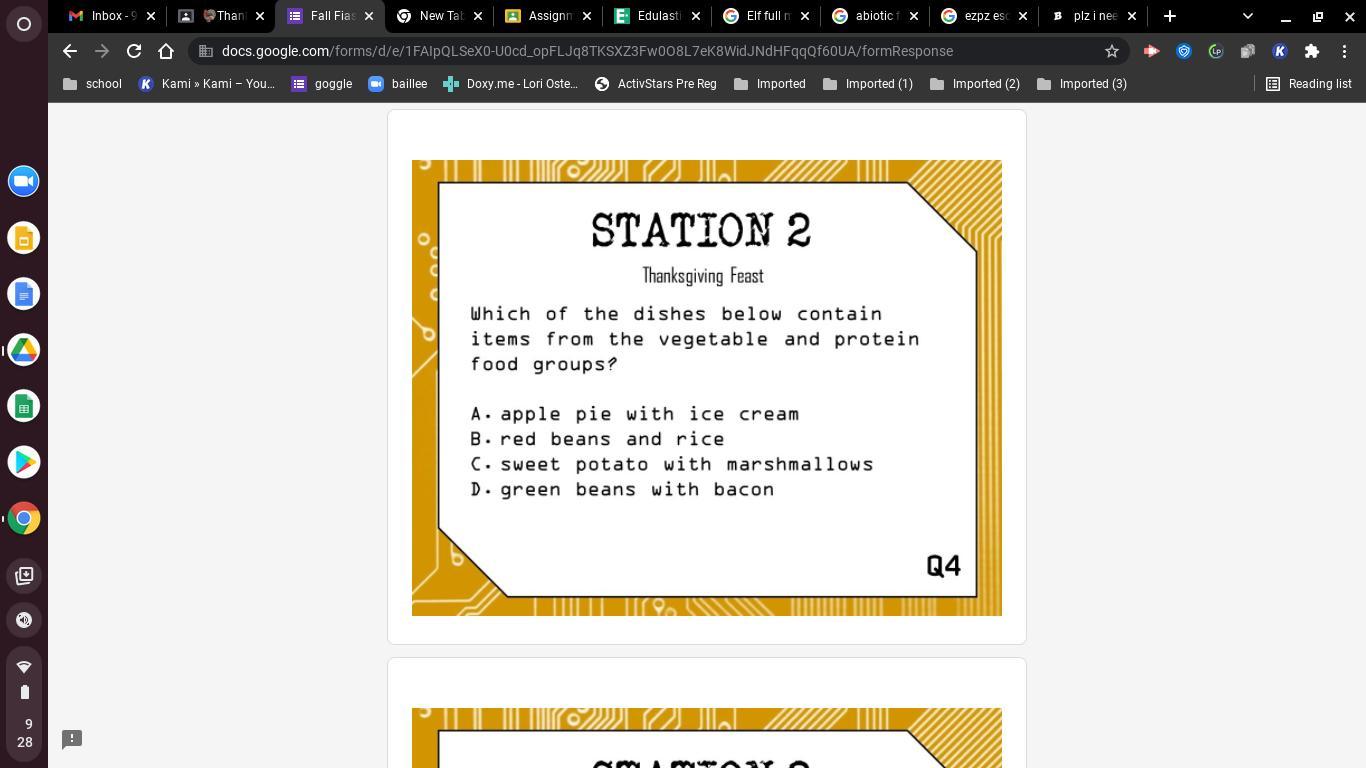

Answers
C is for the digestion questions
B is for the food question
A is the muscle question for stomach
I’m pretty sure
what is the correct classification of a mixture in which both a solid and a liquid are visible?a homogeneous mixture and a solutiona homogeneous mixture and a suspensiona heterogeneous mixture and a solutiona heterogeneous mixture and a suspension
Answers
The correct classification of a mixture in which both a solid and a liquid are visible is a heterogeneous mixture and a suspension.
A heterogeneous mixture is a mixture that has different regions with different compositions and properties.
A suspension is a type of heterogeneous mixture in which the particles of the solid are suspended in the liquid and can be seen with the eye.
In a suspension, the solid particles are not dissolved in the liquid and can settle at the bottom of the container over time if left undisturbed.
In contrast, a homogeneous mixture has a uniform composition and properties throughout the mixture, and the particles are evenly distributed.
Examples of suspensions include muddy water, blood, and paint. In these mixtures, the solid particles are suspended in the liquid and can be seen with the eye.
In contrast, a solution is a homogeneous mixture in which the particles are evenly distributed and cannot be seen with the eye.
In conclusion, a mixture in which both a solid and a liquid are visible is a heterogeneous mixture and a suspension.
to know more about mixture refer here:
https://brainly.com/question/24898889#
#SPJ11
one mole of an ideal gas does 1100 j of work as it expands isothermally to a final pressure of 1.00 atm and volume of 0.027 m3. a). What was the initial volume of the gas, in cubic meters?
b). What is the temperature of the gas, in Kelvin?
Answers
A)the initial volume of the gas was 0.10 m^3. B) The temperature of the gas, in Kelvin t T = 298 K.
Since the process is isothermal, the temperature of the gas remains constant throughout the expansion. Therefore, we can use the following equation to calculate the initial volume of the gas:
W = -nRT ln(Vf/Vi)
where W is the work done by the gas, n is the number of moles of the gas, R is the gas constant, T is the temperature of the gas, Vi is the initial volume of the gas, and Vf is the final volume of the gas.
a) To find the initial volume of the gas, we rearrange the equation as follows:
Vi = Vf exp(-W/nRT)
Substituting the given values, we get:
Vi = (0.027 m^3) exp(-1100 J / (1 mol x 8.314 J/mol-K x 298 K))
Vi = 0.10 m^3
Therefore, the initial volume of the gas was 0.10 m^3.
b) Since the process is isothermal, the temperature of the gas remains constant at T = 298 K.
Learn more about temperature here:
https://brainly.com/question/12035620
#SPJ11
the elements boron, silicon, germanium, and arsenic be classified into? *
Answers
2. The three sub-atomic particles that make up the atom are
Answers
Answer:
Protons
Neutrons
Electrons
Explanation:
How the new competences and skills can make the researcher more successful in their long-term career whether within or outside academia
Answers
By continually acquiring new competences and skills, researchers expand their knowledge base, enhance their problem-solving capabilities, and increase their professional versatility. These attributes make them more adaptable, competitive, and successful in their long-term careers, whether within academia or in other sectors.
Acquiring new competences and skills can significantly enhance a researcher's long-term career success, both within and outside academia. Here are some ways these new competences and skills can contribute to their success:
Adaptability: Developing a mindset and skills that enable researchers to adapt to new technologies, methodologies, and interdisciplinary approaches is crucial. The ability to embrace change and learn new things positions researchers to stay relevant and tackle emerging challenges effectively.Communication and Collaboration: Enhancing communication skills, both written and verbal, enables researchers to effectively convey their ideas, findings, and research outcomes to diverse audiences. Collaboration skills foster productive teamwork, facilitate knowledge sharing, and open up opportunities for interdisciplinary research and innovation. Project and Time Management: Proficiency in project and time management allows researchers to plan, organize, and prioritize their work effectively. This skill ensures that research projects are completed efficiently, deadlines are met, and resources are utilized optimally. Data Analysis and Interpretation: Gaining expertise in data analysis techniques, statistical tools, and data visualization enhances researchers' ability to derive meaningful insights from complex datasets. It enables them to present their findings in a compelling manner, making their research more impactful. Entrepreneurial Mindset: Developing an entrepreneurial mindset helps researchers identify opportunities for innovation, commercialization, and collaboration with industry. It equips them with skills in intellectual property management, grant writing, and project funding, enabling them to translate their research into practical applications. Leadership and Mentoring: Developing leadership skills enables researchers to lead research teams, foster a positive research culture, and inspire others. Mentoring skills allow them to guide and support early-career researchers, contributing to their professional growth and fostering a collaborative research environment.Learn more about competences and skills here:
brainly.com/question/25769973
#SPJ11
The label on an aerosol spray can contains a warning that the can should not be heated to over 130 °F because of the danger of explosion due to
the pressure increase as it is heated. Calculate the potential volume of the gas contained in a 500.-mL aerosol can when it is heated from 25 °C to 54
°C (approximately 130 °F), assuming a constant pressure.
Answers
Answer:
Explanation:
To solve this problem, we can use the ideal gas law, which relates the pressure, volume, temperature, and number of moles of a gas:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature in Kelvin.
First, we need to convert the initial temperature of 25 °C to Kelvin:
T1 = 25 °C + 273.15 = 298.15 K
Then, we can use the initial conditions to find the number of moles of gas:
n = PV/RT1
where we can assume that the pressure is atmospheric pressure (1 atm) and R is the ideal gas constant (0.0821 L·atm/mol·K).
n = (1 atm)(0.5 L)/(0.0821 L·atm/mol·K)(298.15 K) = 0.0204 mol
Next, we can use the final temperature of 54 °C (327.15 K) to find the final volume:
V2 = nRT2/P
V2 = (0.0204 mol)(0.0821 L·atm/mol·K)(327.15 K)/(1 atm) = 0.551 L
Finally, we can subtract the initial volume from the final volume to find the potential volume increase:
ΔV = V2 - V1 = 0.551 L - 0.5 L = 0.051 L
Therefore, if the aerosol can is heated to 54 °C, the potential volume of the gas contained in the can would increase by approximately 0.051 L. However, this increase in volume would cause a corresponding increase in pressure, which could lead to an explosion if the can is not designed to withstand the increased pressure.
A Lewis symbol that has a non-noble gas element and no dots represents a:
Select the correct answer below:
cation
anion
neutral atom
depends on the element
Answers
A Lewis symbol that has a non-noble gas element and no dots represents a neutral atom.
Valence electrons are represented by the dots or dashes around a Lewis symbol. When a Lewis symbol is devoid of dots, the atom is in a neutral state, which means it has an equal amount of protons and electrons, and it also has its complete complement of valence electrons.
By adding dots to the outer energy around the element's symbol to indicate electrons, a Lewis symbol is created. The number of dots for many common items matches the element's group number. The Lewis Symbols for several elements are listed below. Take note of the relationship to each element's group number.
To know more about valence electrons:
https://brainly.com/question/31264554
#SPJ4
A non-noble gas element symbol represented in Lewis symbols with no dots is a cation, as the lack of dots indicates the loss of valence electrons, transforming the atom into a positively charged ion.
Explanation:In the context of Lewis symbols, a non-noble gas element symbol with no dots identifies a cation. In Lewis symbols, dots represent valence electrons, and non-noble gas elements without any valence electrons have lost their outer electrons, becoming positively charged cations. It's important to note that noble gases naturally contain eight valence electrons, so a Lewis symbol with no dots would not accurately represent a noble gas. Instead, it's more likely to represent a non-noble gas that's formed a cation.
Learn more about Lewis symbols and cations here:https://brainly.com/question/32311821
#SPJ11
It consists of two lobes that contain pollen sacs and pollen grains.
Answers
Answer:
Assuming you need the names :)
Explanation:
It's dithecous anther
A bottle is filled with a small amount of a volatile liquid and sealed. Sometime later it is observed that no liquid is evident in the sealed bottle. Which of the following statements would explain this observation? a. More time is needed to establish equilibrium. b. Liquid and vapor are at equilibrium in the bottle. c. Too little liquid was added to achieve a liquid vapor equilibrium in the closed system d. The vapor state is favored when equilibrium is established e. The liquid has undergone sublimation
Answers
The statement that would best explain the observation of no liquid being evident in the sealed bottle is: b. Liquid and vapor are at equilibrium in the bottle.
When liquid and vapor are at equilibrium in a closed system, it means that the rate of condensation (liquid turning into vapor) is equal to the rate of vaporization (vapor turning into liquid). In this case, it appears that all the liquid has vaporized, and no liquid is evident. This suggests that the liquid and vapor have reached a state of equilibrium, where the amount of liquid remaining is negligible compared to the amount of vapor present.
The vapor state is favored when equilibrium is established because the pressure exerted by the vapor phase reaches a point where it equals the vapor pressure of the liquid at that temperature. At this equilibrium point, no further net condensation or vaporization occurs, resulting in the absence of visible liquid in the sealed bottle.
To learn more about equilibrium click here: brainly.com/question/29627805
#SPJ11
If f
1
is 200hz and f
3
is 400hz. What can you say about I
3
? I 3 is an overtone frequency f
3
is a hamonic of f
1
Both AsB Noither A8B QUESTION 10 A string is 1 meter long and has a wave generator that cretaes waves moving at v=20 m/. Wich of the following are NOT standing wave harmonics this string is capable of producing? 10H
2
20 Hz 15 Hz 30 Hz
Answers
The overtone frequency I3 cannot be determined based solely on the given information.
The main answer is that the overtone frequency I3 cannot be determined based solely on the given information. In order to determine the overtone frequency, we need additional information about the specific characteristics of the wave system or the string being analyzed.
The information provided states that f1 is 200 Hz and f3 is 400 Hz. However, without knowing the relationship between these frequencies or the nature of the wave system, we cannot make any conclusive statements about the overtone frequency I3. It is important to note that the terms "overtone frequency" and "harmonic" have specific meanings in the context of wave systems and harmonics.
Learn more about Overtone
brainly.com/question/14471389
#SPJ11
is Gatorade a compound or an element
Answers
Answer:
Element...follow me and mark me brainliest
Answer:
A compound because it cannot be found on the periodic table
Explanation:
Above which temperature does the thermal conductivity of water start to fall?

Answers
Answer:
It’s actually 130 degrees Celsius.
Explanation:
The pH of a solution is 7. Which best describes the solution?
A. The solution has more hydrogen ions than hydroxide ions.
B. The solution has fewer hydrogen ions than hand soap.
C. The solution has the same number of hydrogen ions as apple juice.
D. The solution has the same number of hydrogen ions and hydroxide ions.
Which term best describes a solution with a pH of 5? A. acidic
B. neutral
C. colorless
D. basic
Which best describes the pH scale?
a. Acids measure below 7.
b. Bases measure below 7.
c. Acids and bases measure above 7.
d. Bases and acids measure at 7.
Answers
Answer:
1=B
2=A
3=A
Explanation:
Any subsatance with a ph of less than 7 is acidic.
Bases measure over 7
What charge would an ion have if it had more electrons than protons?
A. It would have a net positive charge.
OB. It would have no charge.
C. It would have a net negative charge.
Ο Ο
D. It would be neutral, since electrons would be outside the nucleus.
Answers
Answer:
it will have a net negative charge and it is known as anion