What is the unit of temperature on a solubility graph
Answers
Answer:
Khfxgkhxxkfhkugxxiufxhfixiyf
Explanation:
Xlufy8fxtukuxfxtu
Answer: Celsius
Explanation:
Related Questions
Water is made of polar molecules, and oil is made of nonpolar molecules. Based on your observations, which molecules attract each other more strongly, polar molecules or nonpolar molecules? Explain your reasoning.
Answers
Based on observation of polar and non-polar molecules, polar molecules would attract each other more strongly.
What are polar and non-polar molecules?Polar molecules are those molecules that can ionize or dissociate into their respective positive and negative ions when in an aqueous solution.
Nonpolar molecules, on the other hand, are not ionic i.e. not dissociating into ions when dissolved in water.
Polar molecules like water (H2O) contains an uneven distribution of cations (+) and anions (-), hence, would be more attracted to one another than nonpolar molecules that have an even distribution of ions.
Learn more about polarity at: https://brainly.com/question/16106487
#SPJ1
Periodic Table: Which families are highly reactive? Which family is unreactive?
Answers
The families that have the highest reactivities are the a lkali metals and earth alkali metals . These are located far left on the periodic table. They are highly reactive because they have an shell with only 1 electron and need to complete the shell to become stable. The family that is unreactive is inert elements or noble gases.
The alkali metals (lithium family) are highly reactive while the noble gases (helium family) are highly unreactive.
What are alkali metals?The alkali metals are the elements of group 1 in the modern periodic table which are lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr).
All alkali metal has their outermost electron in the s-orbital with the outer configuration ns¹. The alkali metals easily lose the outermost electrons to form cations with charge + 1. Therefore they are highly reactive and are stored under oil to stop the reaction with air.
The noble gases have completely filled electronic configurations. The noble gases have a general outer configuration ns²np⁶ so they have completely filled valence shell therefore, they do not feel any attraction for other atoms. Therefore, noble gases exist in monoatomic form. Therefore, noble gases are highly unreactive and also known as inert gases.
Learn more about alkali metals, here:
brainly.com/question/18153051
#SPJ2
Why can we not use the Nitrogen that we breath in
Answers
Answer:
Nitrogen is an inert gas — meaning it doesn't chemically react with other gases — and it isn't toxic. But breathing pure nitrogen is deadly. That's because the gas displaces oxygen in the lungs. Unconsciousness can occur within one or two breaths, according to the U.S. Chemical Safety and Hazard Investigation Board.
Answer:
because it is an insert gas and it is very dangerous for ear and our nose
587. mL of 0.00531 M NaI (aq) is combined with 840. mL of 0.00536 M Pb(NO3)2 (aq). Determine if a precipitate will form given that the Ksp of Pbl2 is 1.40x10-8.
a. Precipitation will not occur because Qsp > Ksp
b. Precipitation will occur because Qsp > Ksp
c. Precipitation will occur because Qsp = Ksp
d. Precipitation will not occur because Qsp < Ksp
e. Precipitation will occur because Qsp < Ksp
Answers
The formation of a precipitate is possible when the product of the ionic concentrations exceeds the Ksp value. Qis is the reaction quotient, which is the ionic product (IP) in a solution.
To determine whether a precipitate will occur, the reaction quotient (Qis) must be compared to the solubility product constant (Kip). The correct option is (d) Precipitation will not occur because Qis < Kip. The calculations are provided solution below; Qis = [Pb2+] [I–]2Moles of NaI = 0.587 L × 0.00531 mol/L = 0.00313 mol Moles of Pb(NO3)2 = 0.840 L × 0.00536 mol/L = 0.00451 mol[Pb2+] = 0.00451 mol / (0.587 L + 0.840 L) = 0.00327 M[I–] = 0.00313 mol / (0.587 L + 0.840 L) = 0.00226 MQsp = (0.00327 M) × (0.00226 M)2 = 1.72 × 10–8 Kip = 1.4 × 10–8As Qsp is less than Ksp, a precipitate will not form. Therefore, the correct option is (d) Precipitation will not occur because Qis < Ksp.
learn more about solution here.
https://brainly.com/question/1616939
#SPJ11
Calculate the mass of 9.75x1021 atoms of helium.
Answers
Answer: 99.5475
Hope this helps(:
what are the rules for rounding off numbers ?
Answers
According to the rules of rounding off, if the number that we are rounding off if following by digits 5 below, we round the number up and if it the follow up digit is 5 or above, we round the number up.
Any particular number can be rounded to the nearest ten, to the nearest hundred, to the nearest thousand, and so on.
According to the rules of rounding off, if the number that we are rounding is followed by a digit that is equal to or greater than 5, that is, 5, 6, 7, 8, or 9, we round the number up. For example, 47 rounded off to nearest ten is 50.
And, if the number that we are are rounding is followed by a digit which is lower than 5, that is, 0, 1, 2, 3, or 4, then we round off the number down. For example, 22 rounded off to the nearest ten is 20.
To know more about round off here
https://brainly.com/question/17353491
#SPJ4
Use the periodic table to answer the questions below.
Which diagram shows the correct electron
configuration for nitrogen (N)?
N N N1
1s
2s
2p
NN ILL
1s 2s
2p
N N
1s
2s
DONE✔
2p

Answers
The diagram that show the correct electron configuration is the second option. ⬆⬇1s, ⬆⬇2s, ⬆⬆⬆2p.
What is electronic configuration?The electronic configuration is the representation of electron present indifferent energy levels is an atom. The different type of orbitals are s, p, d, and f.
Thus, the correct option is 2, ⬆⬇1s, ⬆⬇2s, ⬆⬆⬆2p.
Learn more about electronic configuration
https://brainly.com/question/14283892
#SPJ1
What reaction does a vitamin KH2-dependent enzyme catalyze? View Available Hint(s)
O It decarboxylates the y-carbon of a glutamate side chain.
O It carboxylates the y-carbon of an aspartate side chain.
O It carboxylates the ycarbon of a glutamate side chain.
O It carboxylates the ycarbon of a glutamine side chain.
O It carboxylates the P-carbon of a glutamate side chain.
Submit PartQ CoASH is used to activate carboxylic acids. What type of compound is formed between CoASH and a carboxylic acid? View Available Hint(s)
O anhydride
O thioether
O amide
O thioester
O mixed anhydride with phosphate
Submit ▼ Part H What role does ATP play in biotin-dependent enzymes?
O It puts a good leaving group on bicarbonate.
O It provides a strong base.
O It provides a strong nucleophile.
O It delocalizes electrons It reduces bicarbonate.
Submit Request Answer Part F TPP is the coenzyme required by pyruvate decarboxylase. What product is formed in that reaction? View Avallable Hint(s)
O acetaldehyde
O butanal
O formaldehyde
O acetone
O propanal
Submit
Answers
The glutamate side chain's gamma carbon is carboxylated by Q1, a vitamin KH2 dependent enzyme, and Q2. A thioester is created when carboxylic acid and coASH interact.
Thioesters are organosulfur compounds having the functional group RSC(=O)R'. By virtue of the thio- prefix, they are implied to be comparable to carboxylate esters (ROC(=O)R'), with the sulphur in the thioester acting as the linking oxygen in the carboxylate ester. Thioesters play a crucial role in metabolism. A notable illustration of this is the metabolism of fatty acids. The main metabolite, acetyl coa, is an ester that is predominantly generated when pyruvate undergoes oxidative decarboxylation or when B acids are broken down.
To learn more about carboxylic acid please click on below link
https://brainly.com/question/4721247
#SPJ4
Why is health needed for developed?
Answers
Answer:
Improvised health has been one of the main benefits of development. As humans, we need the primary aspects of life to keep us going. (food, water, oxygen) But scientificly we need health that will improve health. and Improved Health has been one of the main benefits of development. This benefit results partly from an increase in income and partly from scientific progress in the fight against disease and disability.
In your own words, explain why it is necessary to include only one chain-terminating/ synthesis-terminating nucleotide in each well of the electrophoresis instrument.
Answers
Answer: DNA sequencing methods employ chain termination nucleotides. Furthermore, if two chain-terminating nucleotides are employed in the same well, determining which strand is ended by which dideoxynucleotide will be impossible.
(its my own words, but i did search it to get this answer, hope it helps!)
Learn more about chain-terminating here:
https://brainly.com/question/3476061
#SPJ2
A cylinder contains 10 mol of gas at STP. Calculate the temperature and pressure of the gas?
Answers
Temperature is 273 Kelvin and pressure is 1 bar.
Given - 10 mol of gas at STP
Temperature = ?
Pressure = ?
STP - STP or Standard Temperature and Pressure, refers to the nominal atmospheric conditions at sea level. 0 degrees Celsius and 1 atmosphere are the respective temperatures and pressures. The STP value is essential for physicists, chemists, engineers, pilots, and navigators in addition to other specialists.
In order to report on the qualities of matter, standard conditions for temperature (T) and pressure (P) relate to a certain temperature and pressure. STP values are frequently applied in gas experiments.
STP was previously defined as follows by the International Union of Pure and Applied Chemistry (IUPAC):
It is 0 degrees Celsius outside (273.15 degrees Kelvin or 32 degrees Fahrenheit)
One atmosphere (101.325 kilopascal or 760 Torr)
This usage of the term has been dropped. However, the definition of the volume (V) term normal cubic meter continues to be often employed under these circumstances. The IUPAC has used a stricter definition of STP since 1982:
Absolute pressure: 105 Pa or 100,000 pascals (1 bar, 14.5 pounds per square inch, 0.98692 atm).
Hence, Temperature is 273 Kelvin and pressure is 1 bar at STP.
To learn more about STP refer - https://brainly.com/question/27100414
#SPJ9
In which of the following pairs do both compounds have a van't Hoff factor (i) ( i ) of 2 2 ? a. Sodium chloride and magnesium sulfate. b. Glucose and sodium .
Answers
So, sodium chloride and magnesium sulfate have different van't Hoff factors, and they do not have the same van't Hoff factor at a given temperature.For the given pair of substances, the answer is Option (b) Glucose and sodium bicarbonate.
The van't Hoff factor (H) is defined as the change in the concentration of a substance that occurs when its temperature is raised by 1°C, at a constant pressure. The units of the van't Hoff factor are reciprocals of the molar concentration, i.e., 1/M.
For a given substance, the van't Hoff factor can vary depending on the temperature and the concentration of the solution. Therefore, we need to know the van't Hoff factors of both substances to determine if they have the same van't Hoff factor at a given temperature.
To find the van't Hoff factors of the given substances, we can use the following formula:
Δ[A] = [A] * H
where Δ[A] is the change in concentration of substance A, [A] is the initial concentration of substance A, and H is the van't Hoff factor.
Using the given information, we can find the van't Hoff factors for sodium chloride and magnesium sulfate as follows:
For sodium chloride, we have:
Δ[NaCl] = [NaCl] * H = 5.85 * 2.3 = 13.07 mol/L
Using the formula for the change in concentration of a solution, we can find the change in molarity (Δ[M]) of the solution as follows:
Δ[M] = Δ[NaCl] / [NaCl] = 13.07 / 58.5 = 0.22 mol/L
For magnesium sulfate, we have:
Δ[MgSO4] = [MgSO4] * H = 2.3 * 2.3 = 5.85 mol/L
Using the formula for the change in concentration of a solution, we can find the change in molarity (Δ[M]) of the solution as follows:
Δ[M] = Δ[MgSO4] / [MgSO4] = 5.85 / 2.3 = 2.62 mol/L
Learn more about sodium chloride Visit: brainly.com/question/28106660
#SPJ4
The compound pairs that have a van't Hoff factor (i) of 2 are:
a. Sodium chloride (NaCl) and magnesium sulfate (MgSO₄).
Determine the van't Hoff factor?The van't Hoff factor (i) represents the number of particles into which a compound dissociates in a solution. For compounds that do not dissociate, the van't Hoff factor is 1. In the given options, sodium chloride (NaCl) and magnesium sulfate (MgSO₄) both dissociate into ions when dissolved in water.
When NaCl dissolves in water, it dissociates into Na⁺ and Cl⁻ ions. Similarly, MgSO₄ dissociates into Mg²⁺ and SO₄²⁻ ions. Since both compounds dissociate into two ions, the van't Hoff factor (i) for both NaCl and MgSO₄ is 2.
On the other hand, glucose (C₆H₁₂O₆) does not dissociate into ions when dissolved in water, so its van't Hoff factor is 1.
Therefore, glucose and sodium (Na) do not form a compound pair with a van't Hoff factor of 2.
To know more about glucose, refer here:
https://brainly.com/question/13555266#
#SPJ4
electrons tend to occupy the ___________available energy level.
Answers
Electrons tend to occupy the lowest available energy level.
This is in accordance with the Aufbau principle, which states that electrons fill orbitals in order of increasing energy levels. Electrons prefer to occupy lower energy orbitals because they are more stable, and therefore, require less energy to maintain their current state. The electron configuration of an atom describes the arrangement of its electrons in various orbitals.
The energy levels of electrons in atoms are described using the principal quantum number (n). The first energy level (n = 1) is the lowest energy level, and it is closest to the nucleus. As the value of n increases, so does the energy level of the electron, and the distance from the nucleus increases as well. In summary, electrons tend to occupy the lowest available energy level because they are more stable and require less energy.
Learn more about Aufbau principle at:
https://brainly.com/question/15006708
#SPJ11
draw the products formed when phenylacetic acid (c6h5ch2cooh) is treated with the following reagent. socl2
Answers
The product so formed for the given chemical reaction is C₆H₅CH₂COCl.
When phenylacetic acid (C₆H₅CH₂COOH) is treated with SOCl₂, it results into phenylacetyl chloride.
The chemical reaction is depicted as follows-
C₆H₅CH₂COOH + SOCl₂ → C₆H₅CH₂COCl
An acyl chloride (or acid chloride) is an organic compound with the functional group −C(=O)Cl. Their formula is usually written R−COCl, where R is a side chain. They are reactive derivatives of carboxylic acids (R−C(=O)OH). A specific example of an acyl chloride is acetyl chloride, CH₃COCl. Acyl chlorides are the most important subset of acyl halides.
To learn more about carboxylic acids check the link below-
https://brainly.com/question/26855500
#SPJ4
F this car's gas tank holds 45 l , how many tanks of gas will you use to drive 1600 km ?
Answers
1.52 tanks of gas will be used to drive 1600 km.
A certain fuel-efficient hybrid car gets gasoline mileage of 55.0 mpg (miles per gallon).
A gallon is a unit of volume in both the US customary and imperial systems of measurement. The US gallon is defined as 231 cubic inches (3.785 liters).
1 mpg = 0.425143707 km/l; to convert miles per gallon to kilometers per liter .
mileage = 23.38 km/l
volume = 1600 km ÷ 23.38 km/l
volume = 68.43 liters; the amount of gasoline needed the trip
tanks = 68.43 l ÷ 45 l
tanks = 1.52
More about units change: brainly.com/question/26715286
#SPJ4
A refrigerator delays the spoilage of food by ________.
A.killing microorganisms
B.slowing down the rate of chemical reactions within microorganisms
C.expanding the water found within microorganisms
D.diminishing the supply of oxygen to microorganisms
Answers
Please mark brainliest I need 2 more!!!!!!!!!!!
A refrigerator delays the spoilage of food by slowing down the rate of chemical reactions within microorganisms. Therefore, option B is correct.
What do you mean by chemical reaction ?The term chemical reaction is defined as the processes, in which substances undergo a chemical change to formed new substances, with all new properties.
Higher temperatures often result in faster chemical reactions. When food is left on the kitchen counter, it can soon become bad. The process is slowed down by the refrigerator's lower temperature, which allows the same food to stay fresh for longer.
Bacteria, molds, and yeasts are all potential sources of microbial deterioration. Food's water activity plays a significant role in determining the type of food decomposition that results.
Thus, option B is correct.
To learn more about the chemical reaction, follow the link;
https://brainly.com/question/29762834
#SPJ2
trans-1,4-dimethylcyclohexane has two conformations with a difference in energy of 3.6 kcal/mol. what is the cause of this difference? hint: this is twice the difference in methylcyclohexane!
Answers
The cause of the difference in energy between the two conformations of trans-1,4-dimethylcyclohexane is due to steric strain. Steric strain is the repulsion between atoms or groups of atoms that are in close proximity to one another.
About trans-1,4-dimethylcyclohexaneIn the case of trans-1,4-dimethylcyclohexane, the two methyl groups are located on opposite sides of the ring and are in close proximity to one another, causing steric strain and a higher energy conformation. This is twice the difference in methylcyclohexane because there are two methyl groups causing steric strain instead of just one.
The lower energy conformation of trans-1,4-dimethylcyclohexane has the two methyl groups further apart from each other, reducing the steric strain and resulting in a lower energy conformation.
Learn more about conformation at
https://brainly.com/question/17285900
#SPJ11
In which sentence does the verb agree with the subject?
Answers
Answer:
go with that persons
Explanation:
What is the∆S° of 0₂
Answers
Answer:0
Explanation: zero because it is the most stable form of oxygen in its standard state
How do ions in metals behave. There is picture pls help.

Answers
Answer:
metal atoms lose electrons to form positive ions (cations ) non-metal atoms gain electrons to form negative ions (anions )
i agree with this answer from the person above me: Augustlonley
Explanation:
*throws leaves in his face* Hey you wake up!!
what is the melting point for neon
Answers
Answer:
-415.48°F (-248.60°C)
Explanation:
hoped this helped! <3 A brainiest is always appreciated! if this helped give a thanks and rating, i always try to reply to comments! have a nice day :)
Which is not an example of a compound?
Ingnore the top PLEASE HELPP
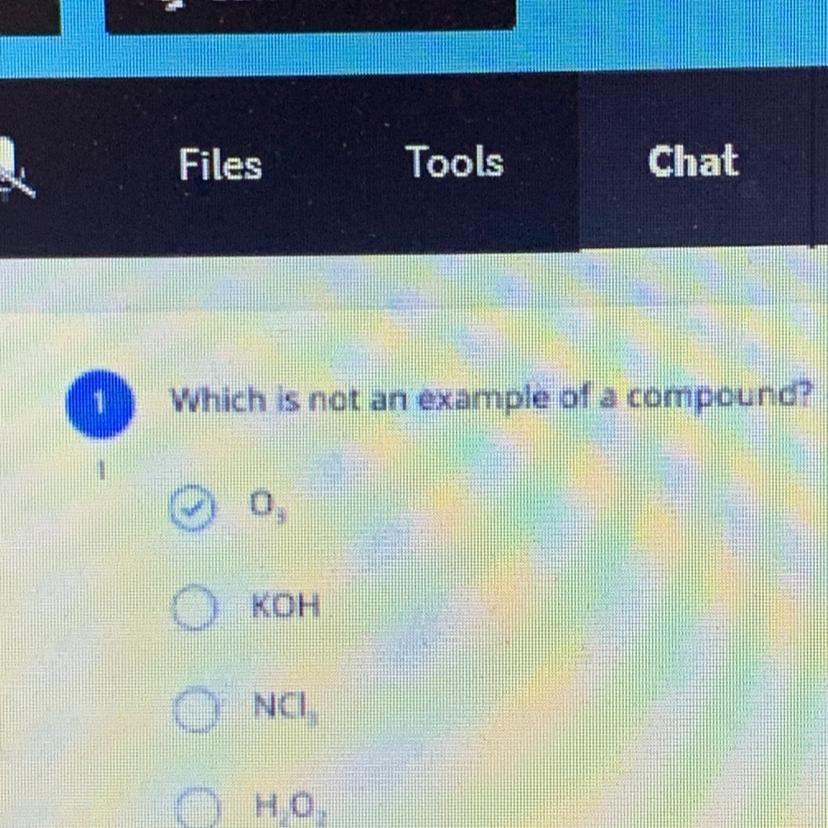
Answers
Answer:
The 3rd answer or NCI is the correct answer
Explanation:
Hope this helps:)
Answer:
the 1st one
Explanation:
please help me i need this good grade

Answers
what charge is a complete atom
Answers
The overall charge of an atom is zero. Atoms are made up of positively charged particles called protons and negatively charged particles called electrons as well as non-charged particles called neutrons
number of moles from the formula is multiplied by the atomic mass of each _______, and then all those masses are added together.
A)
atom
B)
particle
C)
molecule
D)
electron
Answers
Answer:
D. electron
Explanation:
Why do material scientists need to understand chemistry?
A. They change the composition of matter to change the properties
of matter.
B. They make materials that are used by chemists.
OC. They make chemicals that can treat diseases.
D. They measure the forces that are applied to the materials they
make.
Answers
They change the composition of matter to change the properties of matter.
There is matter all around you. All matter is made up of very small particles, including atoms and molecules. The objects you see and touch on a daily basis were constructed from those atoms. Anything that has mass and occupies space (has volume) is considered matter.
The quantity of matter in an item is its mass. A statue made of lead (Pb) or another little object with a lot of mass may be present. You might have a massive object with a small mass.
Additionally, you ought to be aware of the distinction between matter and weight. Weight is a measure of gravity's pull, whereas mass is a measure of the substance of an object.
Thus, They change the composition of matter to change the properties of matter.
Learn more about matter, refer to the link:
https://brainly.com/question/4513444
#SPJ1
A reversed cycle operating as a heat pump uses R-134A as the working fluid. It is designed to operate within the saturation 2 phase vapor-liquid dome with a minimum pressure of 0.700 MPa and a maximum pressure of 1.60 MPa. What is the maximum theoretical Coefficient of Performance of the heat pump
Answers
The max. theoretical Coeff. of Performance of a heat pump operating as a reversed cycle within R-134A saturation phase vapor-liquid dome, with min. pressure of 0.700 MPa and a max. pressure of 1.60 MPa, will be explained.
The Coefficient of Performance (COP) of a heat pump is a measure of its efficiency in transferring heat from a low-temperature reservoir to a high-temperature reservoir. The maximum theoretical COP of a heat pump operating within the given conditions can be determined using the Carnot cycle. The Carnot cycle represents an idealized reversible heat engine and heat pump.
In this case, the heat pump operates within the saturation 2 phase vapor-liquid dome of R-134A, which means that the refrigerant undergoes a phase change from vapor to liquid and vice versa during the cycle. The minimum pressure of 0.700 MPa and the maximum pressure of 1.60 MPa define the operating range of the heat pump.
To calculate the maximum theoretical COP, we need to consider the temperatures of the high-temperature reservoir (TH) and the low-temperature reservoir (TL). The COP is given by the equation\(COP = TH / (TH - TL)\). Since we are only provided with pressure information and not temperature, further data is needed to determine the exact COP value.
Learn more about Coefficient of Performance here:
https://brainly.com/question/28175149
#SPJ11
If you decreased the volume of a sample of gas by a factor of three while maintaining a constant pressure, how would the absolute temperature of the gas be affected?
A gas at 300 K and 4.0 atm is moved to a new location with a temperature of 250 K. The volume changes from 5.5 L to 2.0 L. What is the pressure of the gas at the new location?
What is the partial pressure of 0.50 mol Ne gas combined with 1.20 mol Kr gas at a final pressure of 730 torr?
Answers
**Check for proof photos at the bottom.**
**Answers are in bold.**
__________________________________________________________
If you decreased the volume of a sample of gas by a factor of three while maintaining a constant pressure, how would the absolute temperature of the gas be affected?
✔ it would decrease threefold
A gas at 300 K and 4.0 atm is moved to a new location with a temperature of 250 K. The volume changes from 5.5 L to 2.0 L. What is the pressure of the gas at the new location?
✔ 9.2 atm
What is the partial pressure of 0.50 mol Ne gas combined with 1.20 mol Kr gas at a final pressure of 730 torr?
✔ 215 torr
__________________________________________________________
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
✔ it is decreased to one third of its original volume
Why might a rubber raft burst if it is left in the sun on a summer day?
✔ temperature increases, causing interior pressure to increase
__________________________________________________________
Complete the table by filling in the missing information.
✔ pressure, volume A
✔ temperature, moles of gas B
✔ Charles’s law C
✔ V = kT D
✔ temperature, pressure E
✔ P = kT F
✔ number of moles only G
__________________________________________________________
Match one graph shown at right to each of the gas laws named below.
Charles’s law
✔ d
Gay-Lussac’s law
✔ b
Boyle’s law
✔ e
__________________________________________________________
Left answers:
1.31 atm A
0.44 atm B
Right answers:
✔ decreases it
✔ decreases it
0.12 atm C
__________________________________________________________
Explanation:When decreasing a volume while maintaining the same pressure, the temperature will have to decrease. When volume decreases, particles get squished, increasing pressure. But when temperature decreases, particles move slower, decreasing pressure. The lowered temperature decreases pressure, but the decreased volume increases pressure, so pressure is able to stay constant.
About the 3 main gas laws covered in Edge:
Charles's law says that under a constant pressure, when temperature increases, the volume increases, and vice versa. The equation for this law is V₁/T₁=V₂/T₂.Gay-Lussac’s law says that under a constant volume, when temperature increases, the pressure increases, and vice versa. The equation for this law is P₁/T₁=P₂/T₂.Boyle’s law says that under a constant temperature, when volume decreases, pressure increases, and vice versa. The equation for this law is P₁V₁=P₂V₂.Note that P=pressure, V=volume, and T=temperature. When given a problem, plug in the given values into the correct spots and solve for the unknown value.
Here are photos of Edge. I hope this made your day a little easier.





1)
Give a property belonging to the alkali metal family. (2)
Answers
soft.
silvery.
highly reactive at room temperature
readily lose their outermost electron to form cations with a charge of +1.
identify the importance of water. check all that apply
Answers
Answer
a c e f
Explanation: