What is the identity of the element with an electron configuration of 1s²2s²2p6 3s²3p²? How many
electrons are located in its highest main energy level and what orbitals are they located in?
Answers
Answer:
Aluminum the highest is spdf
Related Questions
EXERCISE 3: WHAT DOES pCO2 CHANGE? - When pCO
2
increases, the concentration of total CO
2
dissolved in water - When pCO
2
increases, the concentration of only CO
2
dissolved in water - When pCO
2
increases, the pH - Which form of dissolved CO
2
is most common in water? Ocean acidification is the decrease in pH due to increasing atmospheric CO
2
concentration.
2
. Choose the correct word option in the statements below: - An organism that needs CO
2
is likely to fare better / worse under ocean acidification. - An organism that needs HCO
3
- is likely to fare better/worse under ocean acidification. - An organism that needs CO
3
2−
is likely to fare better/worse under ocean acidification.
Answers
pCO2 is an important factor that affects various aspects of water chemistry and the impacts of ocean acidification. When pCO2 increases, the concentration of total CO2 dissolved in water also increases. This leads to changes in pH, which decreases due to increasing atmospheric CO2 concentration.
When pCO2 rises, the concentration of only CO2 dissolved in water increases. The dissolved CO2 forms carbonic acid, which contributes to the acidification of the ocean. This increase in CO2 affects the equilibrium between CO2, HCO3-, and CO3^2-, shifting it towards higher levels of dissolved CO2 and H+ ions, resulting in a lower pH.
In terms of the impacts of ocean acidification on different organisms, the effects can vary depending on their specific needs. An organism that requires CO2 is likely to fare better under ocean acidification since the increase in dissolved CO2 can provide them with a favorable environment. However, organisms that rely on HCO3- or CO3^2- may fare worse under ocean acidification, as the lower pH interferes with the availability of these carbonate ions, which are essential for shell formation and calcification in some marine organisms.
To know more about pCO2, click here, https://brainly.com/question/33500517
#SPJ11
How many grams of CO2 are contained in 4.77x10^24 molecules of of CO2?
Answers
Answer:
3.5x1024 3.5 x 10 24 carbon dioxide molecules weighs 256 g.
What type of compound are carbon tetrachloride and boron trihydride?
Answers
Answer:
hydrocarbon
Explanation:
these are called hydrocarbons
I need help with the second question, can anybody help me?

Answers
Answer:
2.66 · 10⁵
Explanation:
There are 1440 minutes in a day. Which means that there is 266400 minutes in 185 days. And in scientific notation it is: 2.66 · 10⁵.
which of the following statements correctly describe a resonance hybrid? select all that apply.multiple select question.the true structure of the resonance hybrid is the structure of the most stable contributor.the resonance hybrid is stabilized due to delocalization of electrons.a resonance hybrid rapidly interconverts between the possible resonance forms.equivalent resonance forms contribute equally to the overall structure of the hybrid.a resonance hybrid has a single structure.
Answers
The most stable contributor's structure is the genuine structure of the resonance hybrid. The delocalization of electrons is what stabilizes the resonance hybrid. The potential resonance forms are quickly interconverted by a resonance hybrid.
Equivalent resonance types equally contribute to the hybrid's overall structure. Consider the resonance hybrid structure of a carboxylate group as an example. Different resonance contributors do not always contribute equally to the hybrid structure until they are equivalent to one another in terms of stability, as is the case for the carboxylate group, which has equivalent contributions from A and B as shown in the given figure. One resonance structure will more closely resemble the “actual” (hybrid) structure than another if it is more stable (lower in energy) than the other.
To know more about resonance hybrid, visit:
brainly.com/question/28318319
#SPJ4

Help me with this please

Answers
= 0.2+560.66JK-2eF
use this app : Photomath
It will help you :)
How many molecules are in 15.0 g of oxygen gas (O2)?
Answers
Answer:
2.82*10^23
Explanation:
please see attached for work!

In the air nitrogen monoxide can react with oxygen gas to form nitrogen dioxide gas, a contributor to photochemical smog.
What is the theoretical yield of nitrogen dioxide gas that can be generated from the reaction of 22.0 g of nitrogen monoxide gas with 22.0 g of oxygen gas? Equation:
LR: ______________
Excess: ___________
Grams of target product produced: _____________
Answers
The balanced chemical equation for the reaction between nitrogen monoxide and oxygen gas is:
2NO(g) + O2(g) → 2NO2(g)
LR: NO
Excess: O2
Grams of target product produced: 30.7 g NO2
StepsThe balanced chemical equation for the reaction between nitrogen monoxide and oxygen gas is:
2NO(g) + O2(g) → 2NO2(g)
To determine the theoretical yield of nitrogen dioxide gas, we need to first identify the limiting reactant (LR) and the excess reactant. To do this, we can calculate the amount of product that would be produced from each reactant, assuming that they completely react:
From 22.0 g of NO: (22.0 g NO) / (30.01 g/mol NO) x (2 mol NO2 / 2 mol NO) x (46.01 g/mol NO2) = 30.7 g NO2
From 22.0 g of O2: (22.0 g O2) / (32.00 g/mol O2) x (2 mol NO2 / 1 mol O2) x (46.01 g/mol NO2) = 64.4 g NO2
Since NO is the limiting reactant, we can expect that 30.7 g of NO2 would be the theoretical yield of the reaction.
LR: NO
Excess: O2
Grams of target product produced: 30.7 g NO2
learn more about theoretical yield here
https://brainly.com/question/25996347
#SPJ1
Determine the mass of Iron (III) oxide (Fe2O3) produced in the decomposition reaction of 100.0 grams of Iron (III) hydroxide (Fe(OH)3).
2Fe(OH)3(s)-1Fe2O3 (s) +3H2O (g)
Answers
Answer: 77.47 g of \(Fe_2O_3\) will be produced from 100.0 g of Iron (III) hydroxide
Explanation:
To calculate the moles :
\(\text{Moles of solute}=\frac{\text{given mass}}{\text{Molar Mass}}\)
\(\text{Moles of} Fe(OH)_3=\frac{100.0g}{106.9g/mol}=0.935moles\)
The balanced chemical equation is:
\(2Fe(OH)_3(s)\rightarrow Fe_2O_3(s)+3H_2O(g)\)
According to stoichiometry :
2 moles of \(Fe(OH)_3\) produce = 1 mole of \(Fe_2O_3\)
Thus 0.935 moles of \(Fe(OH)_3\) will producee=\(\frac{1}{2}\times 0.935=0.468moles\) of \(Fe_2O_3\)
Mass of \(Fe_2O_3=moles\times {\text {Molar mass}}=0.468moles\times 159.7g/mol=74.74g\)
Thus 77.47 g of \(Fe_2O_3\) will be produced from 100.0 g of Iron (III) hydroxide
What is the percent yield if 155 grams of calcium carbonate is treated with 250 grams of hydrichloric acid andb142 grams of calciym chloride is obtained
Answers
Answer:
375 grams
Explanation:
The percent yield if 155 grams of calcium carbonate is treated with 250 grams of hydrochloric acid and 142 grams of calcium chloride is obtained is 82.55%
What is percent yield?Percent yield is defined as the amount of product actually made compared with the maximum calculated yield. It is the ratio of actual yield to the theoretical yield.
Mass of calcium carbonate = 155 g
Mass of HCl = 250g
Mass of calcium chloride product = 142 g
Moles of calcium carbonate = 155 / 100 = 1.55 moles
Moles of HCl = 250 / 36.5 = 6.85 moles
Mass of calcium chloride = Moles x Molar mass
= 1.55 mole x 111 g/ mole
= 172 g /mole
Theoretical yield = 172 g
Actual yield = 142 g
Percent yield = Actual yield / Theoretical yield x 100
= 142 / 172 x 100
= 82.55 %
Thus, the percent yield if 155 grams of calcium carbonate is treated with 250 grams of hydrochloric acid and 142 grams of calcium chloride is obtained is 82.55%
To learn more about percent yield, refer to the link below:
https://brainly.com/question/2506978
#SPJ5
What is the acceleration of a 10kg object if a force of 3N is applied to it
Answers
Answer:
0.3kg/min
Explanation:
The formula for acceleration is a = f/m where f = force and m = mass.
a = 3/10
a = 0.3
Best of Luck
A flat sheet of paper of area 0.130 m2 is oriented so that the normal to the sheet is at an angle of 56 ∘ to a uniform electric field of magnitude 18 N/C .
Answers
The value of electric flux is 2.45N.m²/C.
A flat sheet of paper of area 0.130 m² is oriented so that the normal to the sheet is at an angle of 56 ∘ to a uniform electric field of magnitude 18 N/C.
The electric flux through the paper is given by;
ϕ=E.A.cosθϕ
= Electric fluxE
= Electric field intensityA
= Area of the surfaceθ
= Angle between electric field intensity and normal to the surface
Given,E = 18 N/C A = 0.130 m² θ = 56°
The electric flux through the paper is :
ϕ=E.A.cosθϕ
= (18 N/C)(0.130 m²)cos56°ϕ
= 2.45 N.m²/C
The electric flux through any closed surface is proportional to the charge enclosed by the surface. The electric flux through an open surface can be calculated by multiplying the electric field intensity with the area of the surface and the cosine of the angle between the electric field intensity and the normal to the surface.
To know more about electric field intensity click on below link:
https://brainly.com/question/16869740#
#SPJ11
Complete question:
A flat sheet of paper of area 0.130 m2 is oriented so that the normal to the sheet is at an angle of 56 ∘ to a uniform electric field of magnitude 18 N/C .Find the amount of elctric flux passing through the sheet?
22. What is the difference between lodine-130 and lodine-131?
Answers
The first radioiodine isotopes to be used to treat thyrotoxicosis were iodine-130 and iodine-131 in 1941, and thyroid cancer was added to their list of uses in 1943.
What is Reactor produced radioiodine?Iodine-131, the most frequently prescribed radioiodine isotope for treating thyroid conditions, is a radioactive created in reactors and is widely available in the market. Uranium-235 isotope fission and the so-called (n, ) reaction are its two primary production processes.Iodine-131 is a radioisotope that is simple to obtain in pure form as its chain yield is quite high and radioiodine isotopes with masses higher than 131 have a short half-life. Contrarily, Technetium-130 undergoes a reaction with (n, ) to produce Technetium-131m and Technetium-131g. Depending on whether a wet chemical separation or a dry distillation process is employed, TeO2 or Te-metal is the target material for irradiation.Iodine-131 has a high radiochemical purity and is marketed as a diluted sodium hydroxide solution. When using iodine-131 to identify organic molecules, it may interfere if a certain reducing agent is used to preserve the isotope in the form of iodide in certain solutions.Another radioisotope created in reactors is iodine-125, which is formed when Xenon-124 undergoes the (n, ) reaction. High chemical and radiochemical purity iodine-125 is marketed as a diluted sodium hydroxide solution. It contains 4 to 11 GBq/ml of radioactive material.To Learn more About radioiodine refer to:
https://brainly.com/question/20739702
#SPJ1
how much heat is produced if 7.0 moles of ethane undergo complete combustion?
Answers
The balanced equation for the combustion of ethane, C2H6, is: C2H6 + 3O2 → 2CO2 + 3H2OTo determine how much heat is produced if 7.0 moles of ethane undergo complete combustion, we need to use the balanced equation and the standard enthalpies of formation of the reactants and products.
The standard enthalpy of formation of a compound is the enthalpy change when one mole of the compound is formed from its constituent elements, with all reactants and products in their standard states (usually at 1 atm and 25°C).The standard enthalpies of formation of the reactants and products in the combustion of ethane are:
ΔHf°(C2H6) = -84.68 kJ/mol
ΔHf°(O2) = 0 kJ/mol
ΔHf°(CO2) = -393.51 kJ/mol
ΔHf°(H2O) = -285.83 kJ/mol
Now we can calculate the heat produced by using the difference between the enthalpies of the products and reactants:
2CO2 + 3H2O - (C2H6 + 3O2)
ΔH = 2(-393.51 kJ/mol) + 3(-285.83 kJ/mol) - (-84.68 kJ/mol + 3(0 kJ/mol))
ΔH = -1560.78 kJ/mol
Therefore, if 7.0 moles of ethane undergo complete combustion, the amount of heat produced will be:
-1560.78 kJ/mol x 7.0 mol
= -10,925.46 kJ or -10,925,460 J.
Note that the negative sign indicates that heat is released by the reaction, which is exothermic.
To know more about exothermic visit
https://brainly.com/question/4345448
#SPJ11
What do all of the inventors discussed in this lesson have in common?
1. All of the inventors can be characterized as problem-solvers.
2. All of the inventors used engineering principles to create new things.
3. All of the inventors created new things as children.
4. All of the inventors needed mathematics principles to formulate solutions.
PLEASE I NEED helppp
Answers
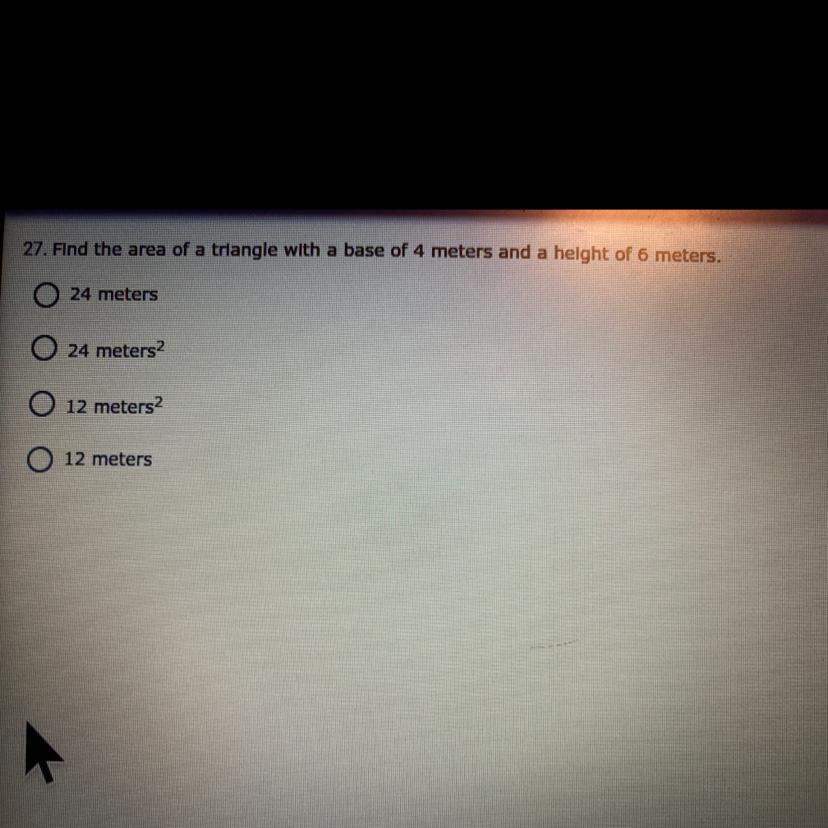
Find the area of a triangle with a base of 4 meters and a height of 6 meters
A.24meters
B.24 meters2
C.12 meters2
D.12 meters
Answers
Answer:
24 meters
Explanation:
24 meter because area
area=Lxw
so just multiply 4 x 6 = 24
Answer:
C. 12 meters2
Which statement best describes a society’s desire for fuel-efficient vehicles? Choose the correct answer.
Auto manufacturers set fuel-efficiency standards.
Interest in fuel efficiency decreases when fuel prices are low.
Fuel shortages in the 1980s started the interest in fuel efficiency.
An increase in fuel efficiency increases carbon dioxide emissions.
Answers
Fuel shortages in the 1980s started the interest in fuel efficiency best describes a society’s desire for fuel-efficient vehicles because of Iran- Iraq war.
Why Fuel shortages in the 1980s started?The Iran-Iraq War in 1980 is the main cause of shortage of oil production. Iraq's and Iran's oil production dropped significantly which triggering economic recessions worldwide.
So that's why society’s desire for fuel-efficient vehicles in order to reduce consumption as well as reduce pollution.
Learn more about fuel here: https://brainly.com/question/25870707
If 3.00 mL of 0.0250 M CuSO4 is diluted to 25.0 mL with pure water, what is the molarity of copper(II) sulfate in the diluted solution
Answers
Answer:
0.00268 M
Explanation:
To find the new molarity, you need to (1) find the moles of CuSO₄ (via the molarity equation using the beginning molarity and volume) and then (2) find the new molarity (using the moles and combined volume). Your final answer should have 3 sig figs to match the given values.
Step 1:
3.00 mL / 1,000 = 0.00300 L
Molarity = moles / volume (L)
0.0250 M = moles / 0.00300 L
(0.0250 M) x (0.00300 L) = moles
7.50 x 10⁻⁵ = moles
Step 2:
25.0 mL / 1,000 = 0.0250 L
0.0250 L + 0.00300 L = 0.0280 L
Molarity = moles / volume (L)
Molarity = (7.50 x 10⁻⁵ moles) / (0.0280 L)
Molarity = 0.00268 M
in an equilibrium reaction with a Keq of 1×10^8,the-
A) reaction are favored
B) reaction is exothermic
C) products are favored
D) reaction is spontaneous
Answers
the isotope has a half life of 5715 years. now there are 40g of the isotope. How much will remain after 1600 years. round to 4 decimal places
Answers
Category ------------ Nuclear chemistry
Sub-category --------- Radioactivity
ANSWER
The remaining amount of isotope after 1600 years is 32.9444 grams
EXPLANATION
Given that;
The half life of the isotope is 5715 years
The initial mass of the isotope is 40 grams
The decay time is 1600 years
Follow the steps below to find the remaining mass after 1600 years
\(\text{ A\lparen t\rparen= A}_o(\frac{1}{2})^{\frac{t}{t_{\frac{1}{2}}}}\)Where
A(t) is the amount of mass remaining after time t
Ao is the initial amount of mass of the substance
t1/2 is the half-life of the substance
t is the decay time
Substitute the given data into the above formula
\(\begin{gathered} \text{ A\lparen t\rparen = 40 \lparen}\frac{\text{ 1}}{\text{ 2}})^{\frac{1600}{5715}} \\ \\ \text{ A\lparen t\rparen = 40 \lparen}\frac{\text{ 1}}{\text{ 2}})^{0.279965} \\ A(t)\text{ = 40 \lparen0.5\rparen}^{0.279965} \\ \text{ A\lparen t\rparen = 40 }\times\text{ 0.8236109} \\ \text{ A\lparen t\rparen = 32.9444 grams} \end{gathered}\)Therefore, the remaining amount of isotope after 1600 years is 32.9444 grams
A 1.5 L pocket of air with a temperature of 295 K rises in the atmosphere. What will be the volume of the air pocket of the t temperature decreased to 2 celsius and the pressure is not changed.
Answers
Answer:
1.4 L.
Explanation:
Applying Charles law,
V/T = V'/T'....................... Equation 1
Where V = Initial volume, V' = Final volume, T = Initial Temperature in Kelvin, T' = Final Temperature in Kelvin.
Make V' the subject of the equation
V' = (V/T)T'..................... Euqation 2
Given: V = 1.5 L, T = 295 K, T' = 2 °C = (2+273) K = 275 K
Substitute these values into equation 2
V' = (1.5/295)275
V' = 1.398
V' ≈ 1.4 L
The car has a rechargeable battery to drive it’s motor. The rechargeable battery provided a potential difference of 330 volts and can store up to 64 mega Jules it takes 8 hours for the battery to receive a full charge assume that the charging process is 100% efficient calculate the total charge the flows while the battery is being charged
Answers
The total charge that flows while the battery is being charged is approximately 193,939.39 Coulombs.
To calculate the total charge that flows while the battery is being charged, we can use the relationship between electrical energy, potential difference, and charge.
The electrical energy (E) stored in the battery is given as 64 mega Jules (64 MJ). The potential difference (V) provided by the battery is 330 volts. We know that the energy (E) is equal to the product of the potential difference (V) and the charge (Q):
E = V * Q
Since the charging process is 100% efficient, all the electrical energy supplied is stored in the battery. Therefore, we can rearrange the equation to solve for the charge (Q):
Q = E / V
Substituting the given values, we have:
Q = 64 MJ / 330 V
To perform the calculation, we need to convert mega Jules (MJ) to joules (J) since the SI unit of energy is joules. One mega Joule is equal to 1 million joules:
Q = (64 * 10^6 J) / 330 V
Calculating the division:
Q ≈ 193,939.39 Coulombs
Therefore, the total charge that flows while the battery is being charged is approximately 193,939.39 Coulombs.
This value represents the quantity of electric charge transferred during the charging process, and it indicates the amount of electricity that enters the battery.
For more such questions on charge visit:
https://brainly.com/question/18102056
#SPJ8
is the isotope a charged atom? explain why or why not
Answers
The isotope is a neutral atom as the number of electrons and protons are equal in an isotopic atom.
What is an isotope?Isotopes can be described as atoms of an element that exhibit the same atomic number but a different atomic mass. Isotopes of an element exhibit an equal number of electrons and protons Therefore, they are not charged atoms. The numbers of neutrons in their respective nucleus of an isotope of an element is different.
In the isotopes of oxygen element, the number of neutrons in oxygen increases by 1, and it creates the isotope of oxygen. The number of electrons or protons in each isotope of oxygen element are eight therefore, all the isotope are neutral.
Therefore, the isotopes of an element are not charged atoms.
Learn more about isotopes, here:
brainly.com/question/11680817
#SPJ1
it is on SWRO plant with a capacity of 50000m3/day the tds of the feed is 41690ppm implying a chloride ion level of around 23000ppm the temperature of the feed is around 18°C in March and 27°C in September the reject has a tds of 64500ppm . the pressure is 70 bar, that plant started to produce water in June 2003 and corrosion problem appeared already few months of service, two type of corrosion could be established, one being crevice corrosion in 11/2" high pressure connector underneath victauling coupling example the same type of problem that have been corrosion in 316L and 317L high pressure piping seven out of 700 such connector were reported to have suffered this type crevice corrosion after 4 months only, provide the remedy to end the problem
Answers
To address the crevice corrosion issue in the high-pressure connectors and piping of the SWRO plant, several remedies can be considered, A SWRO (Sea Water Reverse Osmosis) plant is a water desalination facility that uses reverse osmosis technology to treat seawater or brackish water and produce freshwater
Material Selection: Evaluate the material compatibility with the operating conditions, especially the chloride ion concentration and temperature. Consider using corrosion-resistant alloys such as duplex stainless steel (e.g., 2205) or super duplex stainless steel (e.g., 2507) that have better resistance to chloride-induced corrosion compared to 316L or 317L stainless steel.
Surface Treatment: Apply appropriate surface treatments to enhance corrosion resistance. Passivation or pickling can remove surface contaminants and create a protective oxide layer on the metal surface, reducing the susceptibility to corrosion.
Design Modifications: Evaluate the design of the connectors and piping to minimize crevices and stagnant areas where corrosion can occur. Smooth transitions, avoiding sharp angles or crevices, can help promote better fluid flow and prevent the accumulation of corrosive substances.
Cathodic Protection: Implement cathodic protection methods, such as impressed current or sacrificial anodes, to protect the connectors and piping from corrosion. This technique involves introducing a more easily corroded metal (anode) to the system, which sacrifices itself to protect the connected metal (cathode) from corrosion.
Monitoring and Maintenance: Regularly monitor the corrosion levels and condition of the connectors and piping. Implement a maintenance program that includes periodic inspections, cleaning, and repairs, if necessary, to prevent corrosion from progressing.
It is important to consult with corrosion experts and engineers who specialize in SWRO plant operations to assess the specific conditions, perform material testing, and provide tailored solutions to mitigate the crevice corrosion problem effectively.
To know more about SWRO (Sea Water Reverse Osmosis), click here, https://brainly.com/question/31556553
#SPJ11
Write application of isotopes .
Answers
Answer:
In the chemical industry.
Isotopes are the atoms of the same element with same atomic no: and different mass numbers. ... ⇒As isotope of uranium is used as a fuel in nuclear reactor. ⇒An isotope of iodine is used in the treatment of goitre. ⇒An isotope of cobalt is used in the treatment of cancer.
Which of the following compounds would make a good electrolyte?
Select one:
O a. ethanol
O b. carbon dioxide
O c. sodium chloride
O d. water
Answers
Answer:
C. Sodium Chloride.
Explanation:
Because when NaCl dissolved in water, it will dissociate to produce 2 ions Na+ and Cl-.
The concentration of dye in Solution A is 21.729 M. You have 13 mL of water at your disposal to make the dilutions. The solution is diluted twice, to make Solutions B and C. In the first dilution, 4 parts of Solution A is diluted with 12 parts water to make Solution B. In the second dilution, 6 parts of Solution B is diluted with 4 parts water to make Solution C. What is the concentration of dye in Solution C
Answers
The concentration of dye in solution C was calculated to be 3.839 M.
Dilution is when the extra solvent is added to a solution without increasing the solute concentration. The dilution factor is an expression that describes the ratio of the aliquot to the final volume of the solution. The final solution should be well mixed to make sure that all components are the same.
The dilution factor is a factor used to dilute the stock solution.
Given the concentration of dye in solution A=21.729 M
Solution B- Dilution factor = DF1 = final volume/aliquot volume
DF1 = 16/4 = 4
Solution C- Dilution factor = DF2 = 10/6 = 1.66
So the total dilution factor = DF1 + DF2 = 4 + 1.66 = 5.66
So the concentration of dye in solution C is calculated as
The concentration in solution A/ DF = 21.729/5.66= 3.839 M
To learn more about the solution, refer to the link:
https://brainly.com/question/1616939
#SPJ4
nacl crystallizes in a cubic unit cell with cl- ions on each corner and each face. how many na and cl- ions are in each unit cell of nacl?
Answers
NaCl crystallizes in a cubic unit cell, meaning each side of the cell is equal in length. In this structure, each corner of the cell has a Cl- ion and each face of the cell has a Na+ ion, totaling 8 Cl- ions and 6 Na+ ions per unit cell. So, there are 8 Cl- ions and 6 Na+ ions in each unit cell of NaCl.
NaCl is an ionic compound, meaning it is composed of a metal cation (Na+) and a nonmetal anion (Cl-). Ionic compounds form when electrons are transferred from the metal to the nonmetal, creating a strong electrostatic attraction between the two ions. The oppositely charged ions are then arranged into a lattice structure with a repeating pattern of alternating cations and anions.
The unit cell of a NaCl crystal is a cube, meaning all the sides of the unit cell are of equal length. A single unit cell of NaCl contains eight Cl- ions at the corners and six Na+ ions at the faces of the cube. Since the unit cell of NaCl is symmetrical, the same arrangement of ions is repeated in each adjacent unit cell of the crystal. The arrangement of ions in a NaCl unit cell is important in understanding its properties. Because the cations and anions are arranged in an alternating pattern, the ions form a strong ionic bond. This bond gives the crystal its hardness and stability, making NaCl one of the most common and widely used compounds.
In summary, each unit cell of NaCl contains 8 Cl- ions at the corners and 6 Na+ ions at the faces, forming a cube of equal sides. The alternating arrangement of cations and anions creates an ionic bond which gives NaCl its hardness and stability.
Know more about unit cell here:
https://brainly.com/question/29537966
#SPJ11
what atom has a greater number of neutrons
Answers
Answer:
livermorium
Explanation:
The atom with the largest number of neutrons is a tie between livermorium and tennessine. Each of these atoms contain 177 neutrons.
A train in Japan can travel 813.5 miles in 5 hours
Answers
Answer:
162.7miles/hr
Explanation:
Given parameters:
Distance covered by the train = 813.5miles
Time taken = 5hours
Unknown:
Speed of the train = ?
Solution:
Speed is a physical quantity.
It is mathematically expressed as;
Speed = \(\frac{distance}{time}\)
So, input parameters and solve;
Speed = \(\frac{813.5}{5}\) = 162.7miles/hr