What is one thing that is the same about a mole of sodiums and a mole of carbons?
A) The weight
B) All of these
C) The total number of atoms
D) The mass
Answers
Related Questions
How much energy is required to take a 22. 0-g sample of liquid water at 25. 0 °c to steam at 145. 0 °c?.
Answers
Energy required to take a 22. 0-g sample of liquid water at 25. 0 °c to steam at 145. 0 °c is 58.6 KJ.
q1 = heat required to warm the water from 25.0 °C to 100.0 °C.
= mCΔT
= 22.0 g x 4.184 J∘C−1g−1 x 75
= 6903.6 J
q2 = heat required to vapourize the water to steam at 100 °C.
= mΔH vap
= (22.0 g x 40.7 103 J/ mol )/18 g/ mol
= 49744. 4 J
q3= heat required to warm steam from 100°C to 145.0 °C.
= mCΔT
= 22.0 g x 2.01 J∘C−1g−1 x 45°C
= 1989.9 J
Total energy required to take a 22.0 g sample of liquid water at 25.0 degrees Celsius to steam at 145.0 degrees Celsius is:
= q1 + q2 + q3
= 6903.6 J + 49744. 4 J + 1989.9 J
= 58,637.9 J
= 58.6379 KJ
= 58.6 KJ
Energy is described as having the "ability to conduct work, which is the capacity to apply a force that causes an item to be displaced.
learn more about energy here
https://brainly.com/question/5144470
#SPJ4
What does the term “unicellular” mean? Give an example.
Answers
example-diatoms
Which observation is the best indication that a chemical reaction has occurred?A. Change in temperatureB. Presence of ashesC. Change from liquid to gasD. Absence of fizzing
Answers
Option (A). The best indication that shows a chemical reaction has occurred is the change in temperature.
A chemical reaction occurs when compounds or substances undergo a chemical change to form different compounds or substances. The substance initially involved in a chemical reaction are called reactants. Chemical reactions are usually characterized by a chemical change and they yield one or more products which usually have properties different from the reactants. When a chemical reaction occurs there is certain indication that shows the occurrence of chemical reaction. Those are,
- A change of color occurs during the reaction.
- A gas is produced during the reaction.
- A solid product called a precipitate is produced in the reaction.
- A visible transfer of energy occurs in the form of light as a result of
- Production of an odor
- Change of Temperature
To learn more about Indications of Chemical reaction please visit:
https://brainly.com/question/2390639
#SPJ4
Are protons and electrons stuck together in nuetreal pares or do they orbit closer together around a central nucleus or are they far apart with empty space in between or are they mixed together in a cloud
Answers
Answer:
There is empty space between electrons and protons
Explanation:
The atom is composed of a nucleus and electrons that move round the shells in orbits. The electrons are negatively charged while the protons are positively charged.
In between the nucleus where protons are found and the electrons in orbits is largely empty space, hence the answer above.
Balance the following:
HNO3 + I2 = HIO3 + NO2 + H2O
Answers
answer :
10 HNO3 + I2 = 2 HIO3 + 10 NO2 + 4 H2O
steps
HNO3 + I2 = HIO3 + NO2 + H2O
wikihow
list all elements & number on each side then
match the number
H 1 -> 2
N 1 -> 1
O 3 -> 6
I 2 -> 1
I 2 -> 2 by having 2HIO3
H 1 -> 4
N 1 -> 1
O 3 -> 9
I 2 -> 2
H 4 -> 4
N 1 -> 1
O 9 -> 9
I 2 -> 2
by having
explain law of conservation of mass with an activity
Answers
The law of conservation of mass states that matter cannot be created or destroyed, only transformed from one form to another. This means that the total mass of the reactants in a chemical reaction must equal the total mass of the products.
One way to demonstrate the law of conservation of mass is to conduct a simple chemical reaction in which a solid reactant is transformed into a gas product. For example, you can heat a piece of zinc metal in a test tube and observe the production of zinc oxide gas.
To set up the activity, you will need:
A piece of zinc metal
A test tube
A test tube holder
A bunsen burner
A tripod and wire gauze
Here's how to conduct the activity:
Set up the bunsen burner and place the test tube holder on the tripod.
Place the piece of zinc metal in the test tube.
Place the test tube in the test tube holder.
Light the bunsen burner and adjust the flame so that it is not too hot.
Hold the test tube over the flame until the zinc metal begins to react and produce gas.
Observe the gas being produced and note any changes in the appearance of the zinc metal.
As the zinc metal reacts with the oxygen in the air, it will produce zinc oxide gas. You should see the mass of the zinc metal decrease as it is transformed into gas. However, the total mass of the reactants (zinc metal and oxygen) will be equal to the total mass of the products (zinc oxide gas).
This activity demonstrates the law of conservation of mass, as the total mass of the reactants and products remains constant.
what is the ionic charge lf this atom
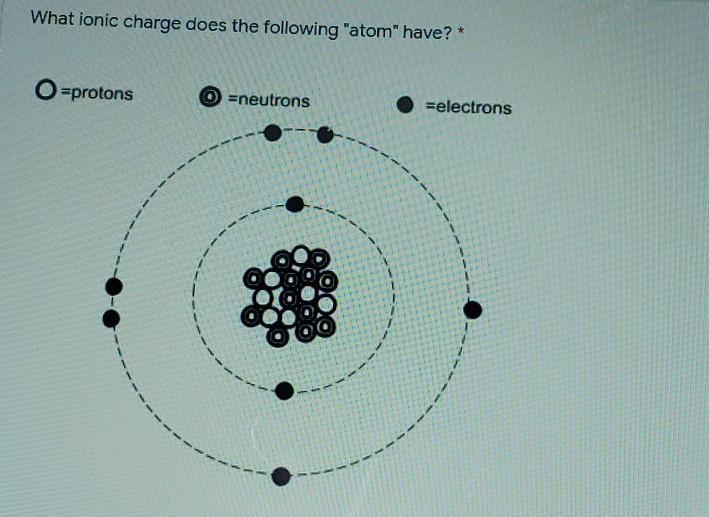
Answers
Answer:
An ionic charge of 2-
Explanation:
The number of electrons is 8 and the number of protons is 2, subtract the two and the difference is the ionic charge. Hope this helps! :)
Answer:
This answer would be 2
Explanation:
The way that I have always done it is you put protons in tally marks (without crossing) over the top of the neutrons and mark them out one by one and whichever one that you have leftover that is your charge.
Hope this helps!
Which of the following is an
example of a homogeneous
mixture?
A. fruit punch
B. a sandwich
C. a toolbox
Answers
Answer:
fruit punch
Explanation:
............
The power of the crane was 68600w Caculate the time taken for the crane to do 3430000 J of work Give the unit
Answers
Answer:
\(T \approx 5s\)
Explanation:
From the question we are told that:
Power \(P=686600w Js^{-1}\)
Work \(W=3430000 J\)
Generally the equation for Time t is mathematically given by
\(T=\frac{Work}{Power}\)
\(T=\frac{W}{P}\)
Therefore
\(T=\frac{3430000 }{686600}\)
\(T=4.996\)
\(T \approx 5 s\)
magnesium trace element which of the following is a trace element, required only in small amounts by most living things?
Answers
A **trace element** required only in small amounts by most living things is **magnesium**.
Magnesium is a vital trace element that plays an essential role in various biochemical processes, including protein synthesis, muscle and nerve function, and maintaining a healthy immune system. Although it is required in small amounts, it is crucial for the proper functioning of organisms. Trace elements like magnesium are needed in lesser quantities compared to macro elements, but their presence is vital for maintaining the overall health and well-being of living organisms. Other examples of trace elements include iron, zinc, copper, and selenium, each playing a unique role in different biological functions.
Know more about trace element here:
https://brainly.com/question/30668185
#SPJ11
How many protons does iron oxide?
Answers
Answer:
26 protons..........
Which atoms have an outer level of electrons that is one electron short of being full? Explain in a paragraph.
Answers
Explanation:
Sorry I tried but I do not know the answer but maybe someone else's know and can tell you the answer
Describe the complete role of the acid catalyst in the dehydration of an alcohol.
Answers
Acid protonates the hydroxyl group and conjugate base deprotonates the beta carbon or adjacent carbon, which leads to formation of a pi bond between the alpha and beta carbon.
What is an acid?
A Brnsted-Lowry acid or Lewis acid is a molecule or ion that may either donate a proton (the hydrogen ion, H+) or establish a covalent bond with an electron pair. Proton donors, also known as Brnsted-Lowry acids, are the first class of acids. Proton donors, sometimes referred to as Arrhenius acids, generate the hydronium ion H3O+ under the unique situation of aqueous solutions. The Arrhenius hypothesis was expanded upon by Brnsted and Lowry to take non-aqueous solvents into account. A hydrogen atom is often bound to a chemical structure in a Brnsted or Arrhenius acid that is still energetically advantageous after the loss of H+.
Acid protonates the hydroxyl group and conjugate base deprotonates the beta carbon or adjacent carbon, which leads to formation of a pi bond between the alpha and beta carbon.
To know more about acids, click on the link
https://brainly.com/question/16986563
#SPJ4
what is the predominant charge of the amino acid abbreviated e at ph 7?
Answers
At pH 7, the amino acid abbreviated as "E" (glutamic acid) is predominantly negatively charged (deprotonated). This is because at pH 7, the carboxyl group (-COOH) of glutamic acid tends to lose a proton (H+), resulting in the formation of a negatively charged carboxylate ion (-COO-).
The amino group (-NH2) of glutamic acid, on the other hand, remains protonated, carrying a positive charge. Therefore, the overall charge of the glutamic acid molecule at pH 7 is negative.
Glutamic acid (abbreviated as "E") is an amino acid that contains both a carboxyl group (-COOH) and an amino group (-NH2) in its structure. At pH 7, which is near neutral, the carboxyl group tends to lose a proton and become deprotonated. This results in the formation of a negatively charged carboxylate ion (-COO-). The amino group, however, remains protonated and carries a positive charge (+NH3+).
The deprotonation of the carboxyl group and the protonation of the amino group are influenced by the pH of the surrounding environment. At pH 7, which is close to the pKa value of the carboxyl group, the majority of glutamic acid molecules will have a negatively charged carboxylate group and a positively charged amino group, resulting in a net negative charge.
In summary, at pH 7, the amino acid abbreviated as "E" (glutamic acid) is predominantly negatively charged.
Learn more about amino acid here: brainly.com/question/31872499
#SPJ11
The predominant charge of the amino acid E at pH 7 is negative.
The predominant charge of an amino acid at a specific pH is determined by the ionization of its functional groups, specifically the amino group (\(NH_2\)) and the carboxyl group (COOH). The pH affects the ionization state of these groups.
Amino acids can be categorized into three groups based on their ionization behaviour:
1. Acidic amino acids: At pH 7, acidic amino acids like glutamic acid (abbreviated as E) have a carboxyl group (COOH) that is ionized and carries a negative charge (COO-). The amino group (\(NH_2\)) remains uncharged.
2. Basic amino acids: Basic amino acids, such as lysine or arginine, have an amino group (\(NH_2\)) that is ionized and carries a positive charge (\(NH_3^+\)). The carboxyl group (COOH) remains uncharged.
3. Neutral amino acids: Neutral amino acids, like glycine or alanine, have both the amino group (\(NH_2\)) and the carboxyl group (COOH) in their neutral, non-ionized forms at pH 7.
In the case of the amino acid abbreviated E (glutamic acid), at pH 7, the carboxyl group (COOH) is ionized and carries a negative charge (\(COO^-\)), while the amino group (\(NH_2\)) remains uncharged.
Therefore, the predominant charge of the amino acid E at pH 7 is negative.
To learn more about predominant charge from the given link
https://brainly.com/question/31035959
#SPJ4
Can someone please help me pls

Answers
Answer:
I guess option d
hope I am correct
What happens when a liquid becomes a gas?
A. Nothing happens.
B. It changes volume but not shape.
C. It changes shape and volume.
D. It changes shape but not volume.
SUBND
Answers
Answer:
Answere-d.
Explanation:
it changes shape but not volume.
How much energy is required to convert 100.0 g of water completely
to steam?
Answers
Answer:
225.6 kJ, assuming the water is already at 100 °C
Explanation:
The correct answer to this question will depend on the initial temperature of the water to which heat is added to produce steam. Energy is required to raise the water temperature to 100°C. At that point, an energy of vaporization is needed to convert liquid water at 100 °C to water vapor at 100°C. The heat of vaporization for water is 2256.4 kJ/kg. The energy required to bring 100g of water from a lower temperature to 100°C is calculated at 4.186 J/g°C. We don't know the starting temperature, so this step cannot be calculated.
Assuming that we are already at 100 °C, we can calculate the heat required for vaporization:
(100.0g)(1000.0g/1 kg)(2256.4 kJ/kg) = 225.6 kJ for 100 grams water.
A student measures the mass of an graduated cylinder to be
14.13 g. They then fill the graduated cylinder with a liquid to a
volume of 19 mL. The mass of the liquid is measured to bbe
59.08 g. An object is then placed in the graduated cylinder.
The volume is then measured to be 41.6 mL. If the final mass
of the cylinder, object, and fluid is measured to be 91.2 g, what
is the density of the object?
Answers
Answer:
Density=189.9/226ml.
Explanation:
total mass=91.2g.
41.6ml-19ml=22.6ml of the object.
mass of the object=91.2-71.21=18.99g.
so D=M/V.
D=18.99/22.6.
D=189.9g/226ml
In a calorimeter, 10.0 g of ice melts at 0oC. The enthalpy of fusion of the ice is 334 J/g.
How much heat was absorbed?
A.) 0.33 kJ
B.) .34 kJ
C.) 33.4 kJ
D.) 334 kJ
Answers
Answer:
D
Explanation:
In the problem above it is
q = mass x heat fusion
q = 10 g x 334 J/g = 3340 J. Since the ice is absorbing heat it is + 334 J.
Answer:
3.34 kJ
Explanation:
took the test

reaction of 1.274 g of aqueous copper sulfate with excess zinc metal produced 0.392 g of copper metal according to the equation
Answers
The mass of aqueous CuSO4 used in the reaction is 0.985 g. As the question states that 1.274 g of CuSO4 was used, this means that there was excess CuSO4 that did not react with Zn.
The given equation is:
CuSO4 (aq) + Zn (s) → Cu (s) + ZnSO4 (aq)
According to the equation, 1 mole of CuSO4 reacts with 1 mole of Zn to produce 1 mole of Cu and 1 mole of ZnSO4. The molar mass of CuSO4 is 159.61 g/mol and the molar mass of Zn is 65.39 g/mol.
First, we need to find the moles of Cu produced:
0.392 g of Cu × (1 mol/63.55 g) = 0.00617 mol
As the reaction is stoichiometric, the same amount of moles of CuSO4 reacts with Zn:
0.00617 mol of CuSO4 reacts with Zn
Now, we need to find the mass of CuSO4 used:
0.00617 mol of CuSO4 × (159.61 g/mol) = 0.985 g
Therefore, the mass of CuSO4 used in the reaction is 0.985 g. As the question states that 1.274 g of CuSO4 was used, this means that there was excess CuSO4 that did not react with Zn.
In conclusion, 1.274 g of aqueous copper sulfate with excess zinc metal produced 0.392 g of copper metal, and the mass of CuSO4 used in the reaction is 0.985 g.
To know more about Aqueous visit :
https://brainly.com/question/30215562
#SPJ11
Cu2(s)+O2(g)=Cu2O(g)+SO2(g)
please help urgent will give brainiest
Answers
Answer:
2 Cu2S + 3 O2 = 2 Cu2O + 2 SO2
Explanation:
2 Cu2S + 3 O2 = 2 Cu2O + 2 SO2
a chemistry student is given four different samples to investigate. the student must determine if each sample is a pure substance or mixture and provide an explanation. which conclusion and explanation are consistent?
Answers
One element or one compound makes a pure substance. A mixture is made up of two or more distinct substances that are not chemically bonded.
Pure Substance can be identified by following characteristics. :
Pure substances are those that are created from a single element or compound.It is a solid, liquid, or gas.Physical characteristics never change.This substance is pure.Physical separation is not a possibility.Constant chemical propertiesExample: Gold, hydrogen gas, and pure waterMixtures can be identified by following characteristics:
Together, many substances and elements make up a combination.It is both homogenous and heterogeneous.Impure physical characteristics.This substance is impure.physical separation by a process It is possible to separate by magnetic separation, evaporation, etc.Chemical characteristics differ.Example: Sand and sugar, oil and water.Pure substances are those that have a fixed makeup and cannot be broken down into its component parts. Elements and compounds are divisions of pure substances. A mixture is any two or more pure substances together. There are two categories of mixtures: heterogeneous and homogeneous mixtures.
To learn more about Pure substance and Mixtures refer here:
https://brainly.com/question/6243623#
#SPJ4
Look at the CHEMICAL EQUATION below...
6H2O + 6CO2 → C6H12O6 + 6O2
Identify the elements used and how many atoms of element are present in the equation.
PRODUCT SIDE REACTANT SIDE
ELEMENTS # OF ATOMS ELEMENTS # OF ATOMS
1. 1.
2. 2.
3. 3.
Answers
Answer:
H=12
O=6+12=18
C=6
and the equation is balanced
Organisms typically have more than one form of each gene. If one form can mask the appearance of another form, that form is considered _______ the other form.
A.
better than
B.
dominant over
C.
recessive to
D.
worse than
Answers
If one form of a gene can mask the appearance of another form, that form is considered dominant over the other form. Option B.
What are dominant alleles?According to Mendel, genes are usually made up of 2 alleles. These alleles can be the same or different. When the alleles are the same, the gene is said to be homozygous. If the alleles are different, the gene is said to be heterozygous.
When the two alleles that make up a gene are different, one will be dominant and the other will be recessive. The dominant gene masks the effect of the recessive gene. In other words, the recessive gene cannot be expressed as long as it coexists with the dominant gene. In order for it to be expressed, it has to be in two copies or a homozygous recessive form.
For the dominant allele, however, only one copy is needed for it to be expressed.
In summary, if one form of a gene can mask the appearance of another form, that form is considered dominant over the other form.
More on genes can be found here: https://brainly.com/question/5519888
#SPJ1
A heat engine has a temperature of 1800 k. some of the heat from the engine flows to the surroundings, which are at a temperature of 160 k. what is the approximate efficiency of the engine? 16% 91% 103% 109%
Answers
The efficiency of the engine is 91%.
The efficiency of the engine signifies how much heat is used and converted successfully into usable heat. It is fact that no engine can be 100% efficient because heat loss takes place in different ways during the process.
The efficiency of the engine is calculated by the formula:
Efficiency = Output heat/Input heat x 100
The temperature of heat engine is 1800 K. The temperature of the surrounding is 160 K.
Substitute the values in the above formula.
Efficiency = (1800 - 160)/1800 x 100
= 91%
Learn more about engine efficiency here:
https://brainly.com/question/2676954
#SPJ4
Answer:
91%
Explanation:
got it right on edge 23
T = 409.5 K, P = 1.50 atm: V = ?L
Answers
Explanation:
T = 409.5 K, P = 1.50 atm: V = 22.4 L The ideal gas law is: PV = nRT where. P = pressure. V = volume n = number of moles.
A solution that has a pH of 5
Answers
Answer:
don't you have any options
A sample of neon gas has a volume of 7.2 mL at a pressure of 1.5atm. What is the pressure exerted by the gas if the volume is increased to 28.8 mL at constant tempature
Answers
The pressure exerted by the neon gas, when the volume is increased from 7.2 mL to 28.8 mL at constant temperature, can be calculated using Boyle's Law. The pressure exerted by the neon gas, when the volume is increased to 28.8 mL at constant temperature, is 0.375 atm.
Boyle's Law states that at constant temperature, the product of the pressure and volume of a gas remains constant. Mathematically, it can be expressed as P₁V₁ = P₂V₂. This law allows us to calculate the change in pressure when the volume changes.
In this case, the initial volume (V₁) is given as 7.2 mL, and the initial pressure (P₁) is 1.5 atm. The final volume (V₂) is 28.8 mL. By substituting these values into Boyle's Law equation, we can solve for the final pressure (P₂).
When we perform the calculations, we find that the pressure exerted by the neon gas, when the volume is increased to 28.8 mL, is 0.375 atm. As the volume increases, the pressure decreases due to the inverse relationship between pressure and volume.
Using Boyle's Law: P₁V₁ = P₂V₂
Given:
Initial volume (V₁) = 7.2 mL
Initial pressure (P₁) = 1.5 atm
Final volume (V₂) = 28.8 mL
To find the final pressure (P₂):
P₂ = (P₁ * V₁) / V₂
= (1.5 atm * 7.2 mL) / 28.8 mL
= 0.375 atm
Therefore, the pressure exerted by the neon gas, when the volume is increased to 28.8 mL at constant temperature, is 0.375 atm.
for such more questions on pressure
https://brainly.com/question/24719118
#SPJ8
underground rock suddenly breaks and there is rapid motion along a fault
Answers
Answer: Earthquakes are usually caused when underground rock suddenly breaks and there is rapid motion along a fault. This sudden release of energy causes the seismic waves that make the ground shake.
Answer: This would cause an earthquake
Explanation:
You have a 0.05 M solution of sulfuric acid. What is the concentration in grams per liter (or dm3)?
Answers
The sulfuric acid solution has a concentration of 4.904 grams per liter (or dm3).
What is sulphuric acid?With the chemical formula H₂SO₄, sulfuric acid is a potent, colorless, odorless, and extremely corrosive mineral acid. It is also referred to as "vitriol oil." An extremely significant industrial chemical, sulfuric acid is used to make a variety of goods, including fertilizers, detergents, pigments, dyes, medicines, and explosives.
The formula below can be used to get the molar mass of sulfuric acid (H₂SO₄):
H₂SO₄'s molar mass is calculated as follows: 2 × (1.008 g/mol) + 32.06 g/mol + 4(16.00 g/mol) = 98.08 g/mol.
Hence, 98.08 g equals one mole of sulfuric acid.
We must multiply the molarity by the sulfuric acid's molar mass in order to translate the molarity (0.05 M) to grams per liter:
4.904 g/L = 0.05 mol/L x 98.08 g/mol
As a result, the sulfuric acid solution has a concentration of 4.904 grams per liter (or dm³).
To know more about sulphuric acid, visit:
brainly.com/question/12986533
#SPJ1