what is kwaishokor?
Answers
Answer:
Kwaishokor is a form of severe protein malnutrition characterized by edema and an enlarged liver with fattty infiltrates.
Explanation:
Related Questions
Which elements are
considered "Noble Metals"?
Answers
Answer:
ruthenium (Ru), rhodium (Rh), palladium (Pd), osmium (Os), iridium (Ir), platinum (Pt), gold (Au), silver (Ag).
Explanation:
The term "Noble Metals" traditionally refers to a group of metals that are resistant to corrosion and oxidation in moist or chemically aggressive environments. The elements commonly considered noble metals are Gold, Platinum, Palladium, Palladium, etc.
Gold is perhaps the most well-known noble metal. It is highly resistant to corrosion and oxidation. Platinum is another widely recognized noble metal. It is extremely resistant to corrosion and has a high melting point.
Palladium is a noble metal that exhibits excellent chemical stability and resistance to corrosion.
To learn more about Noble Metals, follow the link:
https://brainly.com/question/15775417
#SPJ6
What is a buffer made from?

Answers
A buffer is made from an acid - base conjugate pair as shown by option A
What is a buffer?Buffers are often composed of weak acids and their conjugate bases (or weak bases and their conjugate acids). The weak acid can contribute a proton to balance any new base, whereas the conjugate base can absorb a proton to do so.
This balance between the acid and its conjugate base allows the buffer to survive pH changes. Buffers are essential in biological systems because many biochemical processes are particularly sensitive to pH changes.
Learn more about buffer:https://brainly.com/question/31847096
#SPJ1
If the student used 0.7855 g of KHP to standardize 31.22 mL of strontium hydroxide solution, what is the concentration of strontium hydroxide
Answers
The concentration of the strontium hydroxide solution is approximately 0.0616 M.
To determine the concentration of strontium hydroxide (Sr(OH)2) solution, we can use the given information of the mass of potassium hydrogen phthalate (KHP) and the volume of the strontium hydroxide solution. KHP is a primary standard that can be used to standardize a base solution by titration.
The concentration of the strontium hydroxide solution is approximately 0.0616 M.
To determine the concentration, we start by calculating the number of moles of KHP used. By dividing the mass of KHP (0.7855 g) by its molar mass (204.22 g/mol), we find that 0.0038438 mol of KHP was used.
Since the balanced chemical equation between KHP and Sr(OH)2 shows a 1:2 mole ratio, we divide the moles of KHP by 2 to obtain the moles of Sr(OH)2, which is 0.0019219 mol.
Next, we convert the volume of the strontium hydroxide solution (31.22 mL) to liters (0.03122 L). Finally, by dividing the moles of Sr(OH)2 by the volume of the solution, we obtain the concentration of strontium hydroxide, which is approximately 0.0616 M.
Learn more about hydroxide solution.
brainly.com/question/29188985
#SPJ11
If the student used 0.7855 g of KHP to standardize 31.22 mL of strontium hydroxide solution, the concentration of strontium hydroxide is 0.1232 M.
Mass of KHP = 0.7855 g Volume of strontium hydroxide solution = 31.22 ml. It needs to find the concentration of strontium hydroxide.
1. Write the balanced chemical equation for the reaction that occurs between potassium hydrogen phthalate (KHP) and strontium hydroxide (Sr(OH)2)KHC8H4O4 + Sr(OH)2 → K2O + Sr(C8H4O4)2 + 2H2O
2. Calculate the number of moles of KHP used. Molar mass of KHP = 204.23 g/mol. A number of moles of KHP = Mass/Molar mass= 0.7855/204.23= 0.003849 mol.
3. Use the stoichiometry of the balanced chemical equation to find the number of moles of Sr(OH)2. A number of moles of Sr(OH)2 = Number of moles of KHP = 0.003849 mol.
4. Calculate the concentration of Sr(OH)2 in the given solution molarity = Number of moles of solute/Volume of solution in Liters= 0.003849 mol/0.03122 L= 0.1232 M
The concentration of strontium hydroxide is 0.1232 M.
You can learn more about strontium hydroxide at: https://brainly.com/question/32096733
#SPJ11
If the following elements were involved in redox reactions, which noble-gas configuration would they most likely attain
Answers
The noble-gas configurations that the elements would most likely attain in redox reactions are as follows: Lithium (Li) would achieve a helium (He) configuration, Aluminum (Al) and Potassium (K) would attain neon (Ne), Oxygen (O) would attain Ne, Phosphorus (P) would attain argon (Ar), Selenium (Se) could attain either Ar or krypton (Kr), and Strontium (Sr) would attain Kr. These configurations are determined by the tendency of each element to gain or lose electrons in order to achieve a stable electron configuration similar to that of the noble gases.
In redox reactions, elements undergo changes in their electron configurations by either gaining or losing electrons. To determine the noble-gas configuration they would most likely attain, we need to consider their valence electrons and their tendency to gain or lose electrons.
1. Lithium (Li): Lithium has one valence electron and tends to lose it to achieve a stable electron configuration. Therefore, it would most likely attain a helium (He) configuration by losing its valence electron.
2. Aluminum (Al): Aluminum has three valence electrons and tends to lose them to achieve stability. Hence, it would also attain a noble-gas configuration of neon (Ne) by losing all three valence electrons.
3. Oxygen (O): Oxygen has six valence electrons and tends to gain two electrons to achieve stability. It would attain the noble-gas configuration of neon (Ne) by gaining two electrons.
4. Phosphorus (P): Phosphorus has five valence electrons and tends to gain three electrons. Therefore, it would attain the noble-gas configuration of argon (Ar) by gaining three electrons.
5. Potassium (K): Potassium has one valence electron and tends to lose it to achieve stability. Hence, it would attain a noble-gas configuration of argon (Ar) by losing its valence electron.
6. Selenium (Se): Selenium has six valence electrons and can either gain two electrons to achieve a noble-gas configuration of krypton (Kr) or lose six electrons to attain a noble-gas configuration of argon (Ar). Both options are possible depending on the specific reaction conditions.
7. Strontium (Sr): Strontium has two valence electrons and tends to lose them to achieve stability. Therefore, it would attain a noble-gas configuration of krypton (Kr) by losing both valence electrons.
In summary, the noble-gas configurations for the elements are as follows:
Li: He
Al: Ne
O: Ne
P: Ar
K: Ar
Se: Ar or Kr
Sr: Kr
Complete Question : If the following elements were involved in redox reactions, which noble-gas configuration would they most likely attain?
Appropriate elements to their respective bins. ( He OR Ne OR Ar OR Kr ); for { Li, Al, O, P, K, Se, Sr }
Learn more about configuration from the given link https://brainly.com/question/27243888
#SPJ11.
Help needed ASAP, I will mark your answer as brainliest.

Answers
What is the chemical name for the compound IFs?
Select the correct answer.
Answers
if the student calculates that 800000 coulombs of charge were consumed in the production of the 4 moles of hydrogen mentioned above, what is his experimental value for faraday's constant (the charge on one mole of electrons)?
Answers
2.0×10^5 C/mole is his experimental value for faraday's constant if the student calculates that 800000 coulombs of charge were consumed in the production of the 4 moles of hydrogen
Q=nC
Q=charge
n=moles
C= faraday's constant
put values in equation
C=800000/ 4
C=200000=2.0×10^5 C/mole
The amount of electric charge needed to release one gram equivalent of any ion from an electrolytic solution is measured in faraday, also known as the faraday constant, a unit of electricity used in the study of electrochemical processes. It is named for the 19th-century English physicist Michael Faraday and equals 6.022140857 ×10^23 electrons, or 9.648533289 104 coulombs. It is used in electric solutions.
To know more about faraday's constant visit : https://brainly.com/question/24268101
#SPJ4
What are the SEVEN DiAtomic molecules? List them and explain why there are DiAtomic and NOT MonAtomic molecules.
Answers
Answer:
See explanation
Explanation:
The seven elements that form diatomic molecules are:
Hydrogen (H) => H₂
Nitrogen (N) => N₂
Oxygen (O) => O₂
Fluorine (F) => F₂
Chlorine (Cl) => Cl₂
Bromine (Br) => Br₂
Iodine (I) => I₂
How to remember the '7' diatomics => look at periodic table and draw a horizontal line starting at Nitrogen (N) through Fluorine (F), then a vertical line from Fluorine (F) through Astatine (As). The lines drawn make the number '7'. One only need to remember Hydrogen (H) also belongs to the set of elements forming diatomic molecules.

How many protons are pumped in to intermembrane space from matrix by complex iii?
Answers
In chloroplasts, an electron transport chain pumps protons from the stroma to the thylakoid space.
Are protons pumped from matrix to intermembrane space?Inside the chloroplasts are the thylakoids, membranous structures that have embedded an electron transport system and several chlorophyll molecules.When sunlight comes into contact with chlorophyll it causes some of its electrons to gain energy, thus the electronic excitation process encourages them to be transferred to an electron transport chain (system).In chloroplasts there is a fluid called stroma, which has a low concentration of hydrogen ions.When electrons pass through the transport chain from the stroma into the thylakoid lumen, they release their energy generating a concentration gradient of hydrogen ions between the interior of the thylakoid and the stroma.Therefore, we can conclude that the transport of electrons in chloroplasts is the movement of protons from the stroma towards the lumen of the thylakoid, generating a pH gradient on both sides of the thylakoid membrane.To learn more about protons refer to:
https://brainly.com/question/14522409
#SPJ1
The electrons are then transferred to complex III, which also undergoes redox (reduction and oxidation) reactions immediately. This again creates a proton pump, pumping four protons from the matrix through complex III and directly into the mitochondrion's intermembranous space.
What is electrons?The electron is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure.An electron is a negatively charged subatomic particle that together with protons and neutrons form an atom's nucleus.To learn more about electrons refer to:
https://brainly.com/question/860094
#SPJ1
Why do you think there are still smog events when there are pollution
control devices on smokestacks and vehicles?
Answers
Answer:
People just don't really seem to care about how much smog is being polluted anymore. So they still continue to go over the limit of smog and smoke that is supposed to be produced, which cause harm and damage to the environment around us. Hope this helps!
Explanation:
SOMEONE HELP I FEEL LIKE IM RIGHT AM I???

Answers
Comparison between availability of food & population in current scenario.
In UAE.
(10-marks)
100-200 words please
Answers
Answer:
Answer is in the attachment
hope it's helpful

How many ml of 1.0 M NaOH solution are needed to neutralize 100 ml of 2.0 M H2SO4 solution
Answers
Answer:
First you must the balanced equation.
2NaOH+H2SO4 → 2H2O+Na2SO4
From this you can see that it takes 2 moles NaOH per 1 mole H2SO4. Moles H2SO4 = 2.0 mol/L x 0.1 L = 0.2 moles.
Moles NaOH needed = 2 x 0.2 = 0.4 molesVolume NaOH = (x L)(1.0 mol/L) = 0.4 molesx = 0.4 liters = 400 ml NaOH
Explanation:
using the table of thermochemical data, calculate the change in entropy for the formation of diamond from graphite: Cgraphite -> Cdiamond
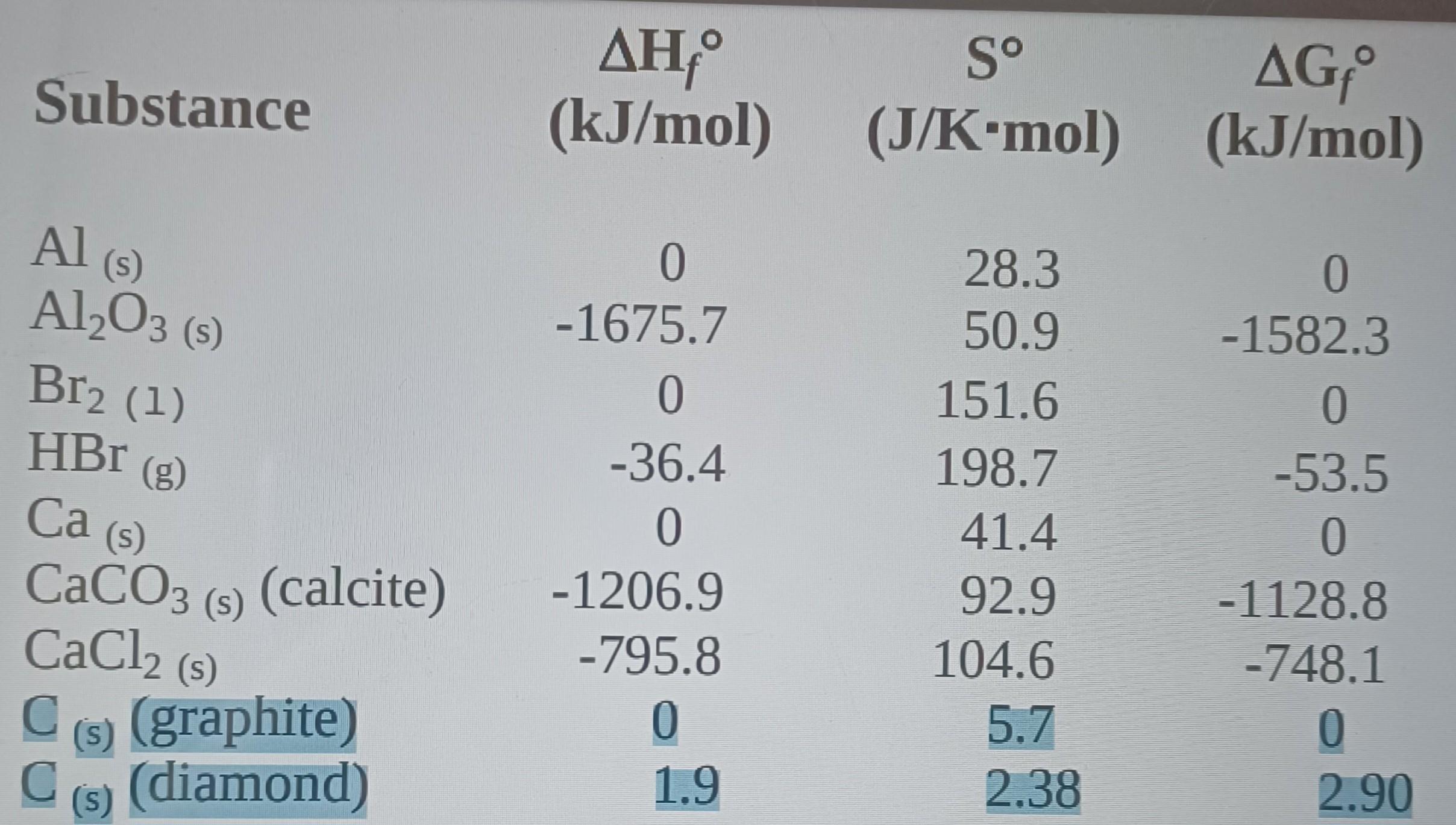
Answers
The change in the entropy is -3.32 J/Kmol
What is the change in entropy?Entropy is a measure of the amount of disorder or randomness in a system. The change in entropy, denoted as ΔS, refers to the difference in entropy between two states of a system.
The change in entropy can be calculated using the equation:
ΔS = S_final - S_initial
where S_final is the entropy of the system in its final state, and S_initial is the entropy of the system in its initial state.
We know that the change in the entropy is obtained from;
Entropy of the products - Entropy of the reactants
2.38 - 5.7
= -3.32 J/Kmol
Learn more about entropy:https://brainly.com/question/13135498
#SPJ1
Why might a coastal region experience more clouds on a warm, sunny day than a desert region?
Answers
Answer:
According to the study, urban heat islands, created from pavement and buildings in big coastal cities like Houston, cause warm air to rise and interact with sea breezes to create heavier and more frequent rainfall in and downwind of the cities.
Which type of plant used light, feathered seeds?
What would a zebra be?
consumer
decomposer
producer
Answers
Answer:
Zebra- consumer
Tiger- consumer
Worm- decomposer
Tree-Producer
Molded bread- decomposer
Plant- producer
Answer:
Type of plant - Dandelion .Zebra is a Consumer .Explanation:
Dandelion plant produces light and feathery seeds .
Zebra consumes / eats grass so it is a consumer .
Match the term to the correct definition.

Answers
Answer:
Vitamin ----- Organic Compounds.Minerals ----- Inorganic Compounds .minerals >>> inorganic
vitamins >>> organic
Explanation:
vitamins are made by plant or animals making them organic substance
minerals haven’t been bonded with carbon and cannot bring life to a cell making them inorganic
hope this helped :)
If 20 hits equal 1 web 1 futz equal 2 geese 10 webs equal to 1 futz How many gits are equal to 5gews?
Answers
Answer:
500 hits
Explanation:
If 20 hits equal 1 web 1 futz equal 2 geese 10 webs equal to 1 futz How many hits are equal to 5 geese?
Solution:
Given that:
20 hits = 1 web, 1 futz = 2 geese; 10 webs = 1 futz
10 webs = 1 futz; hence:
1 web = 1 web * 1 futz / 10 webs = 0.1 futz
1 web = 0.1 futz
1 futz = 2 geese; hence:
0.1 futz = 0.1 futz * 2 geese / 1 futz
0.1 futz = 0.2 geese
Therefore, 1 web = 0.1 futz = 0.2 geese
1 web = 0.2 geese
This means that 20 hits = 1 web = 0.2 geese
20 hits = 0.2 geese
5 geese = 5 geese * 20 hits / 0.2 geese
5 geese = 500 hits
In the Rutherford nuclear-atom model, ________. Group of answer choices mass is spread essentially uniformly throughout the atom the three principal subatomic particles (protons, neutrons, and electrons) all have essentially the same mass and mass is spread essentially uniformly throughout the atom the heavy subatomic particles, protons and neutrons, reside in the nucleus the light subatomic particles, protons and neutrons, reside in the nucleus the three principal subatomic particles (protons, neutrons, and electrons) all have essentially the same mass
Answers
Answer:
the heavy subatomic particles, protons, and neutrons, reside in the nucleus
Explanation:
The atom is envisioned by Ernest Rutherford as miniature solar system, involving electrons that is orbiting around a very massive nucleus, it is regarded as mostly empty space, having nucleus that occupy small part of the atom. He discovered that atom is regarded as mostly empty space, having a mass concentrated central nucleus. This nucleus is charged positively and it is been surrounded by electron which is negatively charged t
Large distance. In In the Rutherford nuclear-atom model,the heavy subatomic particles, protons, and neutrons, reside in the nucleus
What is the specific name of the process for the reaction between the chromate ester and water?
Answers
The reaction between chromate ester and water is a part of a huge and consecutive reaction called Jones reaction.
In this reaction, alcohol is converted to chromic acid in presence of jones reagent, chromic acid is further converted to chromate ester.
Now the obtained chromate ester has one or more unstable alpha hydrogens and in presence of basic species like water it yields an aldehyde or ketone as the organic product.
O O
ll ll
R--R'CH--OH + H-O--Cr--OH ----> R-R'CH-O-Cr--OH +H₂O
ll ll
O O
Alcohol Chromic acid chromate ester
Further, let us understand the reaction of chromate ester with water with an example:
O O
ll ll
(CH₃)CHO---Cr---OH + H₂O ---->(CH₃)CO + Cr--OH +H₃O⁺
ll ll
O O
TO know more about ketone here
brainly.com/question/12308782
#SPJ4
Electron arrangement Carbon
Answers
Carbon has atomic number=Z=6
Let's write electronic configuration
\(\\ \tt\bull\rightarrowtail 1s^22s^22p^2\)
2shells2electrons in 1s orbital.2electrons in 2s orbital2electrons in 2p orbitalConsider a 5.430 g mixture of FeO and Fe3O4. You react this mixture with excess of oxygen to form 5.779 g Fe2O3. Calculate the percent mass of FeO in the original mixture.
Answers
Answer:
Explanation:
A 5.430 g mixture of FeO and Fe3O4 is reacted with excess of oxygen to form 5.779 g Fe2O3. Find the masses of FeO and Fe3O4 present in the mixture.
I found the answer by doing the following:
Fe2O3 = 159.69 g/mol
FeO = 71.745 g/mol
Fe3O4 = 231.535 g/mol
5.779 g Fe2O3 / 159.69 g/mol = 0.03619 mol Fe2O3
x + y :rarrow: 0.03619 mol Fe2O3, where x = FeO and y = Fe3O4.
This implies that y = 0.03619 - x.
5.430 g = x*71.745 g/mol + (0.03619 - x)*231.535g/mol
x = 0.01846 mol * 71.745 g/mol = 1.324 g FeO
y = 0.03619 - 0.01846 = 0.01773 mol * 231.535 g/mol = 4.105 g Fe3O4
My question is why doesn't the following work:
4FeO + O2 :rarrow: 2Fe2O3
4Fe3O4 + O2 :rarrow: 6Fe2O3
_______________________
2FeO + 2Fe3O4 + O2 :rarrow: 4Fe2O3
5.779 g Fe2O3 / 159.69 g/mol = 0.03619 mol Fe2O3
Product:reactants in question are in a 2:1 ratio as given by the stoichiometric coefficients.
0.03619 mol / 2 = 0.01810 mol of FeO and 0.01810 mol Fe3O4.
0.01810 mol FeO * 71.745 g/mol = 1.299 g FeO
0.01810 mol Fe3O4 * 231.535 g/mol = 4.191 g Fe3O4
Obviously this doesn't work because the original mixture is 5.430 g and not 1.299 g + 4.191 g = 5.490 g. Why doesn't this work the way I think it should? Thank you.
how many grams are in 4.07x10^15 molecules of calcium hydroxide
Answers
There are 5.01 × 10-⁷grams in 4.07 x 10¹⁵molecules of calcium hydroxide.
HOW TO CALCULATE MASS:
The mass of a substance can be calculated by multiplying the number of moles in the substance by its molecular mass.
However, given that the number of molecules in calcium hydroxide is 4.07 x 10¹⁵molecules, we need to calculate the number of moles in Ca(OH)2 as follows:
no. of moles of Ca(OH)2 = 4.07 x 10¹⁵ ÷ 6.02 × 10²³
no. of moles = 0.676 × 10-⁸
no. of moles = 6.76 × 10-⁹ moles.
Molar mass of Ca(OH)2 = 74.093 g/mol
Mass of Ca(OH)2 = 74.093 × 6.76 × 10-⁹ moles
Mass = 5.01 × 10-⁷grams.
Therefore, there are 5.01 × 10-⁷grams in 4.07 x 10¹⁵molecules of calcium hydroxide.
Learn more about how to calculate mass at: https://brainly.com/question/8101390?referrer=searchResults
calculate the number of atoms in 2.5 mol manganese.
Answers
Answer:
1.5 × 10²⁴ atoms Mn
General Formulas and Concepts:
Chemistry - Atomic Structure
Reading a Periodic TableUsing Dimensional AnalysisAvogadro's Number - 6.022 × 10²³ atoms, molecules, formula units, etc.Explanation:
Step 1: Define
2.5 mol Mn
Step 2: Identify Conversions
Avogadro's Number
Step 3: Convert
\(2.5 \ mol \ Mn(\frac{6.022 \cdot 10^{23} \ atoms \ Mn}{1 \ mol \ Mn} )\) = 1.5055 × 10²⁴ atoms Mn
Step 4: Check
We are given 2 sig figs. Follow sig fig rules and round.
1.5055 × 10²⁴ atoms Mn ≈ 1.5 × 10²⁴ atoms Mn
Write the word and balanced chemical equations for the reaction between:
Potassium hydroxide + hydrochloric acid
Answers
Answer:
Potassium hydroxide + Hydrochloric acid → Potassium chloride + Water.
potassium hydroxide + hydrochloric acid = neutralisation
the decomposition of nobr to form no and br2 has a rate constant of 5.69 m-1 min-1 at 293 k. if the initial concentration is 1.86 m, what is the concentration of nobr after 3.62 min of reaction?
Answers
The concentration of NOBr using rate law for the decomposition after 3.62 min of reaction is 6.55 x \(10^{-10}\) M.
The rate law for the decomposition of NOBr can be expressed as:
rate = k[NOBr]
where k is the rate constant and [NOBr] is the concentration of NOBr.
Using the given rate constant and temperature, k = 5.69 \(m^{-1}\) \(min^{-1}\) at 293 K.
To find the concentration of NOBr after 3.62 min, we can use the integrated rate law for a first-order reaction:
ln([NOBr]t/[NOBr]0) = -kt
where [NOBr]t is the concentration of NOBr at time t, [NOBr]0 is the initial concentration of NOBr, k is the rate constant, and t is the time elapsed.
Substituting the given values, we get:
ln([NOBr]t/1.86) = -(5.69 \(m^{-1}\) \(min^{-1}\)) x (3.62 min)
ln([NOBr]t/1.86) = -20.64
Solving for [NOBr]t, we get:
[NOBr]t = 1.86 x \(e^{(-20.64)}\)
[NOBr]t = 1.86 x 3.52 x \(10^{-10}\)
[NOBr]t = 6.55 x \(10^{-10}\) M
Learn more about concentration at
https://brainly.com/question/10725862
#SPJ4
Need organic chemistry 11th grade
for practice and 1 question is enough :)
Answers
Answer:
ok all you need is a little study
Explanation:
organic chemistry isn't something to worry about is something that is a very simple concept if you understand what you're doing so a little bit of studying will help you go through it and you really don't this is not the topics that you will really understand if you just go through it as once so there is no need to worry about organic chemistry it is very simple concept just learning you are a you are close just learn all those simple aspects alkane etc it's very easy
Smoking till my eyes roll back like the omen lyrics.
Answers
Use the following equations to determine the heat of the reaction of:
N2H4(l) + H2(g) --> 2NH3(g) ΔH=?
N2H4(l) + CH4O(l) → CH2O(g) + N2(g) + 3H2(g) ΔH = -37
kJ N2(g) + 3H2(g) → 2NH3(g) ΔH = -46
kJ CH4O(l) → CH2O(g) + H2(g) ΔH = -65 kJ
Answers
ebsite uses cookies to ensure you get the best experience on our website. Learn more
Got it!
Doing your Assignments
Doing your Assignments
Sign in
How it works
Examples
Reviews
Homework Answers
Blog
Contact us
Submit
95 982
Assignments Done
98.8%
Successfully Done
In February 2021
Physics help
Math help
Programming help
Answer to Question #96220 in Molecular Physics | Thermodynamics for Kaleesh
Answers>Physics>Molecular Physics | Thermodynamics
Question #96220
a) Determine the enthalpy changes, ΔH for the reaction below, given the following reactions and subsequent ΔH values. Please rewrite the amended chemical reaction equation again.
N2H4 (l) + CH4O (l) CH2O (g) + N2 (g) + 3H2 (g)
2NH3 (g) N2H4 (l) + H2 (g) ΔH = 22.5kJ
2NH3 (g) N2 (g) + 3H2 (g) ΔH = 57.5 kJ
CH2O (g) + H2 (g) CH4O (l) ΔH = 81.2 kJ
b) Given that the enthalpy of vaporization for water as below:
H2O (l) H2O (g) ΔH vap = 44.0 kJ mol-1
Calculate enthalpy ΔH for each of the following processes:
i) Evaporating 3.00 moles of water
ii) Evaporating 3.00 grams of water
i) Condensing 20.0 grams of water
(3 Marks)
c) Use the enthalpy of formation data to calculate the enthalpy of the reaction below.
2C2H6 (g) + 7O2 6H2O (g) + 4CO2 (g)
what volume of bromine trifluoride is required to produce 106 liters of fluorine gas according to the following reaction? (all gases are at the same temperature and pressure.)
Answers
70.6 litres of bromine trifluoride is required to produce 106 liters of fluorine gas according to the following reaction provided that all gases are at the same temperature and pressure.
Since, 2BrF3 (g) gives Br2 (g) + 3 F2 (g)
Here 2 mole BrF3 produces 3 mole F2
If volume produced of F2 is 106 litres
Therefore, volume of BrF3 =?
Since, v1 is directly proportional to n1
Therefore, v1/n1 = v2/n2
v1/2moles = 106 litres/3 moles
v1= 212/3 = 70.6 litres
To learn more about bromine trifluoride visit:
https://brainly.com/question/29037418
#SPJ4