Upon being diagnosed with Cirrhosis of the liver, doctors urge patients to limit the amount of salt intake in their diet to help decrease the rate of scarring. During salt intake the blood becomes increasingly (hypertonic, hypotonic) to the interstitial fluid. Due to this osmotic imbalance, the liver cells become (hypertonic, hypotonic) to the interstitial fluid. This causes the liver cells to appear (shriveled, swollen).
Answers
Upon being diagnosed with cirrhosis of the liver, doctors urge patients to limit the amount of salt intake in their diet to help decrease the rate of scarring. During salt intake the blood becomes increasingly hypertonic to the interstitial fluid. Due to this osmotic imbalance, the liver cells become hypotonic to the interstitial fluid. This causes the liver cells to appear swollen.
What is cirrhosis?Cirrhosis is a liver injury that is generated by various causes in which the liver begins to form fibrous because scars are generated in it. The lesions occur for different reasons, such as excessive alcohol consumption, and the liver will try to heal itself by forming scars, but as this happens, the cirrhosis worsens and can be fatal.
Therefore, we can confirm that upon being diagnosed with cirrhosis of the liver, doctors urge patients to limit the amount of salt intake in their diet to help decrease the rate of scarring. During salt intake the blood becomes increasingly hypertonic to the interstitial fluid. Due to this osmotic imbalance, the liver cells become hypotonic to the interstitial fluid. This causes the liver cells to appear swollen.
To learn more about cirrhosis visit: https://brainly.com/question/8443910
#SPJ1
Related Questions
What is the mass percentage of C in C2H2O2?
Provide an answer to two decimal places.
Answers
\(12 \times 2 + 2 \times 2 + 16 \times 2\)
\( = 24 + 2 + 32 \)
\( = 58 \: gram \: per \: mole\)
Carbon Contribution by mass:\(58 = 100\% \\ 24 = x\%\)
\(x = \frac{24 \times 100}{58} = 41.38\%\)
How will you convert Aniline to Parabromo-Phenol
Answers
Answer:
Aniline is heated with acetic anhydride to form acetanilide. Treatment with bromine/acetic acid gives p-bromoacetanilide. Acid/alkaline hydrolysis gives p-bromoaniline.
Explanation:
Robert was changing the oil in his truck. He dumped the used oil on the ground in his yard. He didn't know it, but Robert was hurting the environment because the oil-
HELP FAST
Answers
Answer:
goes deep into the ground and pollutes the groundwater.
The symbol X in the following equation, 23/11 Na + 1/1H → 23/12 Mg + X, is a/an
Answers
Answer:
X = ¹₀n
Explanation:
The mass number is the total amount of neutrons and protons in the element. The atomic number is the total protons in the element. The mass number is the superscript and the atomic number is the subscript. The mass and atomic numbers must be balanced on both sides of the equation. The element can be identified by the atomic number.
In the event that we have an atomic number of zero, there is no element this represents, so the letter "n" should be used.
²³₁₁Na + ¹₁H → ²³₁₂Mg + X
Mass Number:
Reactants = Products
23 + 1 = 23 + ?
24 = 23 + ?
1 = ?
Atomic Number:
Reactants = Products
11 + 1 = 12 + ?
12 = 12 + ?
0 = ?
The complete equation is:
²³₁₁Na + ¹₁H → ²³₁₂Mg + ¹₀n
what are 2 ways that all hydrocarbons are alike?
Answers
Answer:
Composition: All hydrocarbons are made up of only two types of atoms: carbon and hydrogen. They are like building blocks that contain carbon and hydrogen stuck together.
Organic Nature: Hydrocarbons are special because they are part of a group of compounds that come from living things or things that were once alive. They have carbon and hydrogen in them, which is what makes them different from other types of compounds.
Explanation:
calculate the mass of nickel metal which will react with 25mL of 0.15mol/L (
hydrochloric acid to produce nickel (II) chloride. 0.110625g
Answers
Answer:
0.110625 g of Ni
Explanation:
The first step in solving this problem is to put down the accurate chemical reaction equation.
Ni(s) + 2HCl(aq) ---> NiCl2(aq) + H2(g)
Secondly, we obtain the amount of HCl that reacted from the information provided.
Volume of HCl (V)= 25 ml
Concentration of HCl (C)= 0.15 mol/L
Then, to find the number of moles of HCl (n);
n= CV
Substitution values
n= 25/1000 × 0.15
n= 3.75 ×10^-3 moles
Mass of 3.75 ×10^-3 moles of HCl = number of moles × molar mass
Molar mass of HCl= 36.5 gmol-1
Therefore;
Mass of HCl = 3.75 ×10^-3 moles × 36.5 gmol-1
Mass of HCl= 0.136875 g of HCl
Thirdly we determine the mass of Ni reacted;
If 1 mole of Ni reacted with 2 moles of HCl according to the reaction equation
Then x moles of Ni reacts with 3.75 ×10^-3 moles of HCl
x= 1 × 3.75 ×10^-3 moles/ 2
x= 1.875 × 10^-3 moles of Ni
Mass of Ni= 1.875 × 10^-3 moles of Ni × 59 gmol-1
Mass of Ni= 0.110625 g of Ni
which of the following statement about genes and trait is true
Answers
Answer:
Genes are segments of deoxyribonucleic acid (DNA) that contain the code for a specific protein that functions in one or more types of cells in the body.
Chromosomes are structures within cells that contain a person's genes. A trait is any gene-determined characteristics and is often determined by more than one gene.
for study island:
A single trait can be controlled by multiple genes.
A single gene can influence multiple traits.
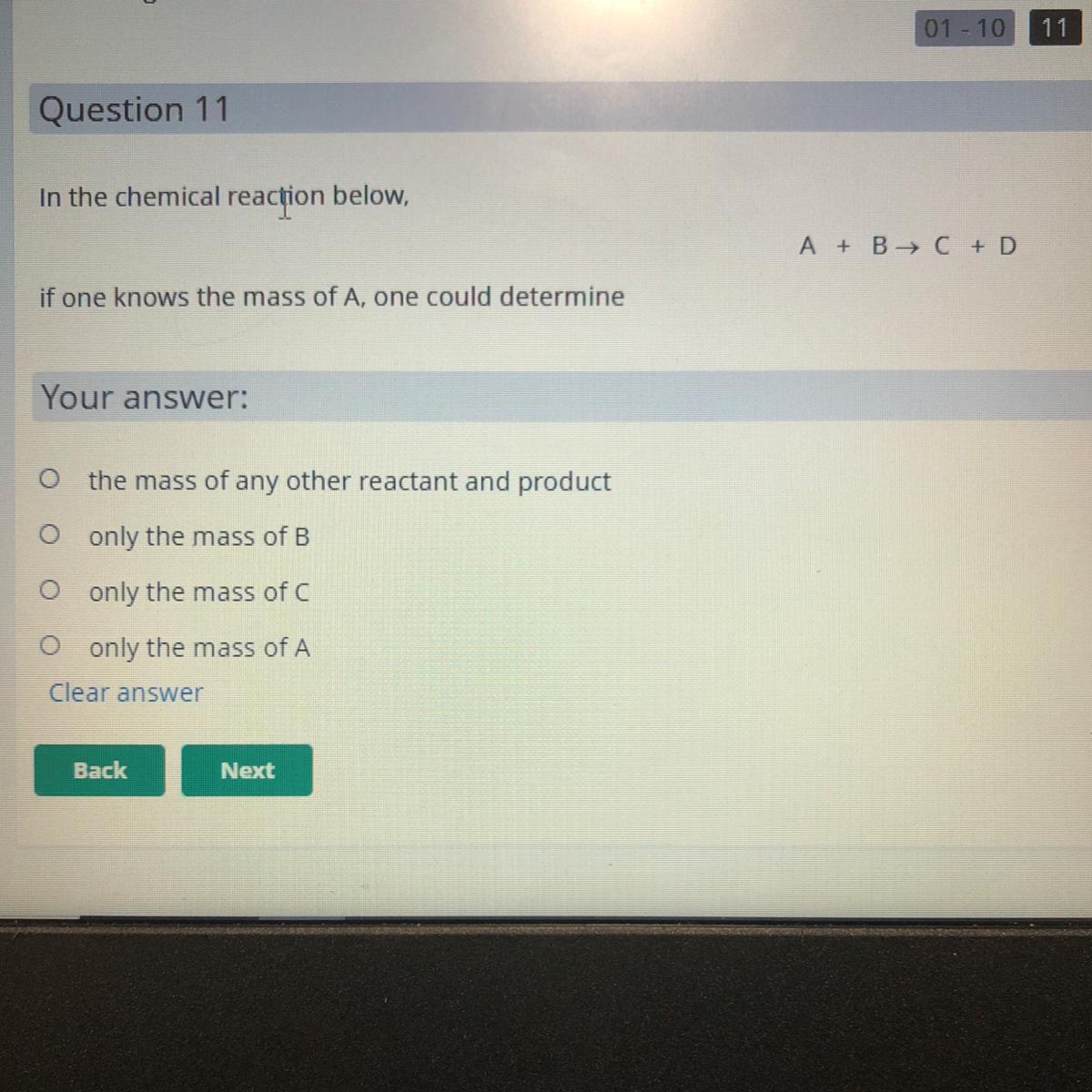
In a chemical reaction, if one knows the mass of A, one could determine:
1) the mass of any other reactant and product
2) only the mass of B
3) only the mass of C
4) only the mass of A
Answers
If one knows the mass of A, they may calculate the mass of any other reactant and product in a chemical reaction.
How can the mass relationship in a chemical reaction be determined?Find out how many moles each reactant has. To identify which reactant is limiting, compare the mole ratios of the reactants with the ratio in the balanced chemical equation. Determine how many moles of the limiting reactant can be converted into the product.
When the mass of the limiting reactant is known, how does one calculate the mass of the product?Calculate the maximum number of moles of product that can be produced from the limiting reactant using mole ratios. Add the product's molar mass to the number of moles.
To know more about chemical reaction visit:-
brainly.com/question/29762834
#SPJ1
Experiment 4: A chemist mixes aqueous solutions of sodium hydroxide and aluminum chloride in a double-displacement reaction, which forms a white solid precipitate and a clear solution. Write the complete, balanced molecular equation for the reaction. Include physical states.
balanced equation:
Answers
The balanced molecular equation for the reaction between sodium hydroxide (NaOH) and aluminum chloride (\(AlCl_3\)) in aqueous solution can be written as follows: 2NaOH(aq) + 3\(AlCl_3\)(aq) → 3NaCl(aq) + \(Al(OH)_3\)(s)
In this reaction, sodium hydroxide (NaOH) reacts with aluminum chloride (\(AlCl_3\)) to form sodium chloride (NaCl) and aluminum hydroxide (\(Al(OH)_3\)). The coefficients in the balanced equation indicate the stoichiometric ratio between the reactants and products.
The physical states of the substances are indicated by the symbols (aq) for aqueous solutions and (s) for the solid precipitate.
The reaction is a double-displacement reaction, also known as a precipitation reaction. Double-displacement reactions involve the exchange of ions between two compounds, resulting in the formation of a precipitate.
In this case, sodium hydroxide and aluminum chloride react to form sodium chloride and aluminum hydroxide, with aluminum hydroxide being the white solid precipitate.
It's worth noting that the actual reaction might involve hydrated forms of the compounds, such as NaOH·x\(H_2O\) and \(AlCl_3\)·y\(H_2O\). However, for simplicity, these hydrated forms are not included in the balanced equation.
Overall, the balanced equation represents the chemical reaction between sodium hydroxide and aluminum chloride, showing the reactants, products, and their stoichiometric ratios.
For more such question on balanced molecular equation visit:
https://brainly.com/question/11904811
#SPJ8
Calculate the concentration in mol/L, M, of an aqueous sugar solution with a concentration of 5.0% (w/w) and density of 1.000 g/mL at 25°C . The molecular weight of sugar is 342.30 g/mol. Report your answer to three significant figures.
Answers
The concentration of the solution is 0.3 mol/L.
What is the concentration in mole per liter?We know that concentration is the amount of substances that is present. We know that the concentration can be measured by the use of several units and one of the units that we can be able to use is the mol/L.
We have that the concentration in w/w is 5.0% and this means that the mass of water in the solution is 5g. From the density of the solution;
volume = mass/density
= 5g/ 1.000 g/mL
= 5 mL or 0.005 L
Number of moles of sugar = 5g/342.30 g/mol
= 0.015 moles
Concentration = Number of moles/volume
= 0.015 moles/0.005 L
= 0.3 mol/L
Learn more about concentration:https://brainly.com/question/10725862
#SPJ1
oxidation number of Ag in Ag2O
Answers
The oxidation number of Ag in Ag2O is +1.
In Ag2O, there are two silver atoms (Ag) and one oxygen atom (O). Oxygen is known to have an oxidation number of -2 in most compounds. Since the compound is neutral, the sum of the oxidation numbers of all the atoms must equal zero.
Therefore, the oxidation numbers of the two silver atoms must add up to +2 to balance out the -2 oxidation number of the oxygen atom. Since there are two silver atoms, each silver atom must have an oxidation number of +1 to yield a total oxidation number of +2 for the compound.
In Ag2O, the silver atoms lose one electron each to form Ag+ ions. This results in an oxidation number of +1 for each silver atom. The oxygen atom gains two electrons from the silver atoms to achieve a stable octet configuration, resulting in an oxidation number of -2 for the oxygen atom. The compound Ag2O is formed through the transfer of electrons, with each silver atom exhibiting an oxidation number of +1.
for such more questions on oxidation
https://brainly.com/question/13182308
#SPJ8
why do metals tend to lose electrons to form positive ions; nonmetals tend to gain electrons to become; these elements are nonmetals that gain one electron to form 1 ions; do nonmetals tend to gain or lose electrons; metals tend to lose electrons to become positive ions; do metals gain or lose electrons to form ions; metals tend to lose electrons and become; why do metals lose electrons
Answers
In a reaction between two different types of materials, metals often lose electrons to finish out their octet whereas non-metals receive electrons to do the same.
A definition of an element.A crucial component of a whole. a simple material that cannot be divided into smaller components or transformed into another substance is referred to as in chemistry. Atoms, which are made up of protons, neutrons, and electrons, are the building blocks of an element. One element has a fixed number of protons in each of its atoms.
What does a simple word "elements" mean?A material is considered to be an element if all of its atoms contain the same number of protons, or, to put it another way, if all of the atoms. The most basic chemical forms are those of the elements.
To know more about Elements visit:
https://brainly.com/question/13025901
#SPJ4
Solve this organic transformation....use - Br2,CCl4,KOH,CH3OH,Hg+2,diluted H2SO4, PCC,HBr,Mg,Dry ether,Na,H2,Pd,quinoline
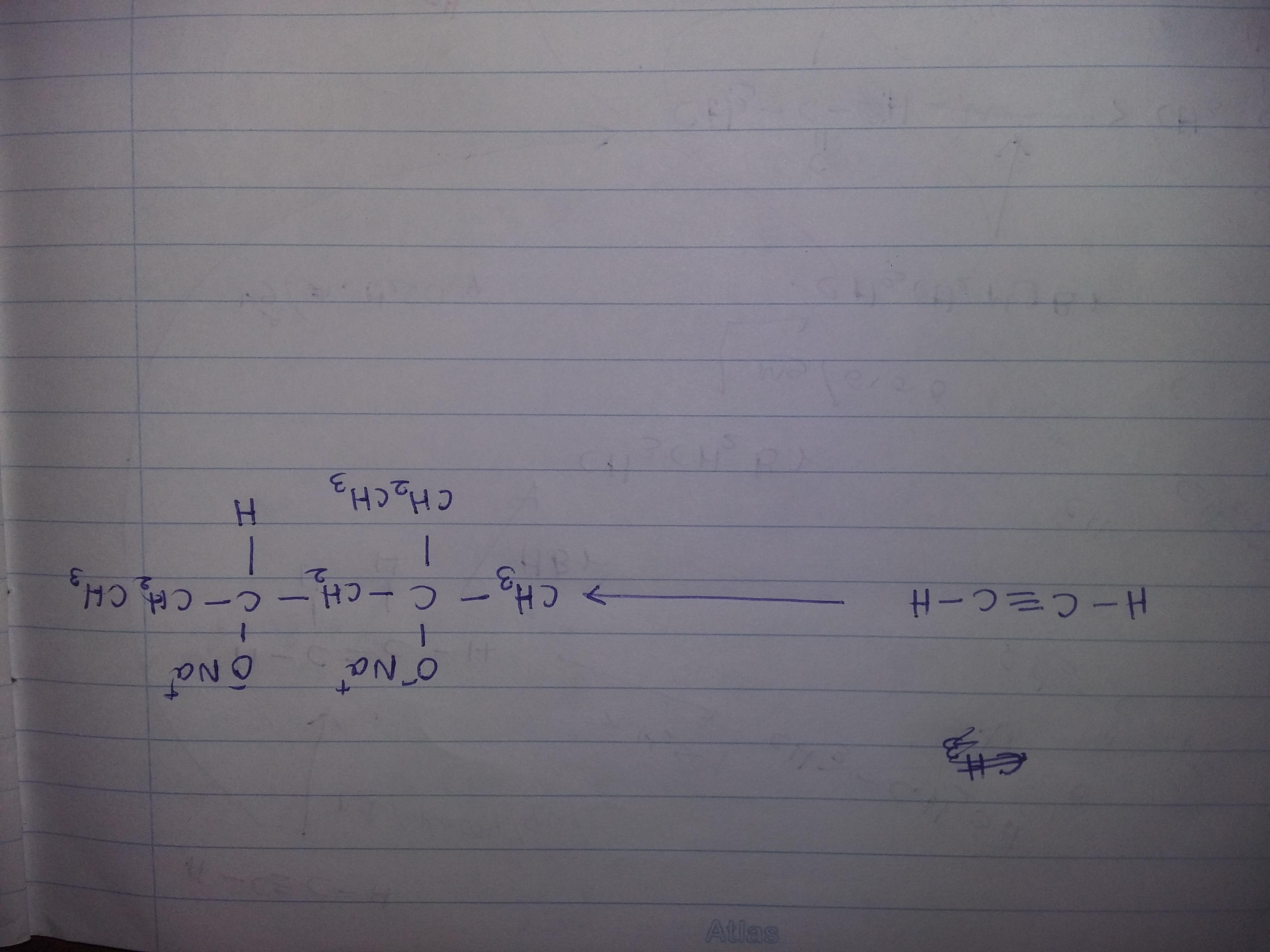
Answers
Organic transformation sequential equation using catalysts will be as follows:
2CH3-CH2-O => (alc. KOH) => CH2=CH2 + KCl + H2O => (Br2/CCl4) => CH2Br-CH2Br + Zn
CH2Br-CH2Br + Zn => (HBr /Pd) => CH2=CH2+ZnBr2
As can be visualized from above organic transformation equation, conversion of dry ether in presence of alkaline potassium hydroxide results in formation of unstable ethene. This dry unsaturated compound of ethene is stabilized by reaction that happens in presence of bromine or calcium tetrachloride as the catalyst which results in formation of ethylene bromide which in presence of highly efficient palladium as catalyst results in formation of stable ethene as byproduct. Thereby with formation of stable compound of ethylene, it releases zinc bromide as byproduct resulting completion of reaction equation. This stable product ethene is a double bonded carbon structure that is chemically extremely flammable and has planar structure.
To know more about organic transformation:
brainly.com/question/14413579
#SPJ1
Calculate the number of C atoms in 0.716 mole of C?
Answers
Answer:
The answer is 4.31 × 10²³ C atoms
Explanation:
The number of C atoms can be found by using the formula
N = n × Lwhere n is the number of moles
N is the number of entities
L is the Avogadro's constant which is
6.02 × 10²³ entities
From the question we have
N = 0.716 × 6.02 × 10²³
We have the final answer as
4.31 × 10²³ C atomsHope this helps you
Course Activity: Oxidizing and Reducing Chemicals (CHEMISTRY EDMENTUM)
TASK 2 PART C:
-Compare the mass of the zinc strip from test tube 5 with the mass of the same strip measured in task 1, part A. Based on your observations of the metal strip, explain what factors may have added to its mass or caused a decrease in its mass. Which had the greater effect? Explain your reasoning.
PLEASEE GIVE A GOOD RESPONSE I'M SO LOST
Answers
The electrolytic reaction between Zn and Ag results in the oxidation of Zn and reduction silver metal. The oxidation of Zn make the Zn ions pass into the solution and its mass decreases.
What is electrochemical reaction ?An electrochemical cell converts chemical energy to electrical energy. It involves redox reaction of two metallic electrodes. The electrode with higher negative electrode potential undergo oxidation and one with lower negative or having positive potential undergoes reduction.
The electrode which undergo oxidation is consumed in the process and metal gets ionized. Ions move into the electrolyte. Whereas, the ions of the second electrode gets reduced into metallic state and deposits on the electrode.
In this case, Zn is oxidizing and it is consumed to reduce the silver metal on the cathode. Therefore, the mass of Zn metal decreases in the strip.
Find more on electrochemical cells:
https://brainly.com/question/22433727
#SPJ1
For an aqueous solution of sodium chloride (NaCl), determine the molarity of 3.95 L of a solution that contains 143 g of sodium chloride.
-Determine the volume of this solution that would contain 3.93 moles of sodium chloride.
-Determine the number of moles of sodium chloride in 20.55 L of this solution.
Answers
Answer:
1.) 0.619 M
2.) 6.34 L
3.) 12.7 moles
Explanation:
Part 1: To find the molarity, you first need to convert grams to moles using the molar mass.
Molar Mass (NaCl): 22.990 g/mol + 35.453 g/mol
Molar Mass (NaCl): 58.443 g/mol
143 grams NaCl 1 mole
------------------------- x ------------------------ = 2.45 moles NaCl
58.443 grams
Molarity = moles / volume (L)
Molarity = 2.45 moles / 3.95 L
Molarity = 0.619
Part 2: To find the volume, you need to use the given moles and the previously calculated molarity.
Molarity = moles / volume (L)
0.619 M = 3.93 moles / volume
(0.619 M) x volume = 3.93 moles
volume = 6.34
Part 3: To find the moles, you need to use the given volume and the previously calculated molarity.
Molarity = moles / volume (L)
0.619 M = moles / 20.55 L
12.7 = moles
what is the need to refer to books before using appreciable amount of chemicals
Answers
Answer:
Prudent execution of experiments requires not only sound judgment and an accurate assessment of the risks involved in the laboratory, but also the selection of appropriate work practices to reduce risk and protect the health and safety of trained laboratory personnel as well as the public and the environment. Chapter 4 provides specific guidelines for evaluating the hazards and assessing the risks associated with laboratory chemicals, equipment, and operations. Chapter 5 demonstrates how to control those risks when managing the inventory of chemicals in the laboratory. The use of the protocols outlined in Chapter 4 in carefully planned experiments is the subject of this chapter.
Explanation:
How can you tell the mass was conserved in a chemical reaction?
Answers
Answer:
\(\boxed {\boxed {\sf Mass \ of \ products \ = \ mass \ of \ reactants}}\)
Explanation:
The Law of Conservation of Mass states that mass cannot be created or destroyed.
In a chemical reaction, there are products and reactants. The reactants react and produce the products.
Since mass can't be created or destroyed, the mass of the products must be equal to the mass of the reactants if mass was conserved in a chemical reactions.
Which statement best describes the relationship between ocean CO2 and pH?
Question 3 options:
When CO2 increases, pH increases and acidity decreases.
When CO2 increases, pH decreases and acidity increases.
When CO2 increases, pH decreases and acidity decreases.
There is no relationship between CO2 and pH
Answers
Answer:
The answer is When CO2 increases, pH decreases and acidity increases.
Explanation:
Excess carbon dioxide (CO2) in the atmosphere gets absorbed at the ocean surface of the ocean. This excess CO2 results in more hydrogen ions, which increases the acidity of the ocean.
How many moles of hydrogen atoms are there in 43g of pentathol C5H11OH
Answers
Answer:
Using your answer from Part A, calculate the volume of a mole of Na atoms (in cm3/mol ). Assume that the entire volume is occupied by Na atoms leaving no gaps or holes between adjacent atoms. (Answer A is V= 2.70*10^7)
Calculate the AHrxn from the AH of formation for the following reaction. C2H4(g) + 302(g) 2C02(g) + 2H20(1). Formation AH values for C2H4(g) = 52.30 kJ/mol, for 02(g) = 0 kJ/mol, for CO2(g) = -393.5 kJ/mol and for H20(1) =-285.8kJ/mol.A. -1305kJB. 1350kJC. 1411kJD. -1411kJ
Answers
Answer
A. -1305 kJ
Explanation
Given:
Equation: C2H4(g) + 302(g) ---- > 2C02(g) + 2H20(l).
Formation ΔH values:
for C2H4(g) = 52.30 kJ/mol,
for 02(g) = 0 kJ/mol,
for CO2(g) = -393.5 kJ/mol, and
for H20(1) = -285.8kJ/mol.
What to find:
The ΔHrxn from the ΔH of formation for the given reaction.
Step-by-step solution:
ΔHrxn = (Sum of ΔH formation for the product) - (Sum of ΔH formation for the reactants).
ΔHrxn = (ΔH for 2CO2(g) + ΔH for 2H2O(l)) + (ΔH for C2H4(g) + ΔH for 3O2(g))
ΔHrxn = [(2 x -393.5) + (2 x -285.8)] + [52.30 + (3 x 0)]
ΔHrxn =(-787.0 - 571.6) + (52.30 + 0)
ΔHrxn = -1358.6 + 52.30
ΔHrxn = -1306.3 kJ
so the closest answer is A. -1305 kJ
Which solids are insoluble in water.
Answers
Some types of solids that are insoluble in water are:
Metals. (most of them)Non-Metallic ElementsMetal OxidesSome Non-Metallic ElementsMetal Carbonates (most of them)Metal Sulfides (most of them)Salts (some of them)Which solids are insoluble in water?Many solids are insoluble in water, meaning they do not dissolve in water to a significant extent. Here are some examples of common solids that are generally insoluble in water:
Metals: Most metals, such as gold, silver, platinum, and copper, are insoluble in water.
Non-Metallic Elements: Many non-metallic elements, such as carbon (in the form of graphite or diamond), sulfur, phosphorus, and iodine, are insoluble in water.
Metal Oxides: Some metal oxides, particularly those of less reactive metals, are insoluble in water. Examples include aluminum oxide (Al2O3), iron(III) oxide (Fe2O3), and lead(II) oxide (PbO).
Metal Carbonates: Most metal carbonates are insoluble in water. Examples include calcium carbonate (CaCO3), lead(II) carbonate (PbCO3), and copper(II) carbonate (CuCO3).
Metal Sulfides: Many metal sulfides are insoluble in water. Examples include lead(II) sulfide (PbS), silver sulfide (Ag2S), and mercury(II) sulfide (HgS).
Insoluble Salts: Certain salts have limited solubility in water. Examples include silver chloride (AgCl), lead(II) iodide (PbI2), and calcium sulfate (CaSO4).
It's important to note that while these solids are generally insoluble in water, they may exhibit some solubility to a small extent. The solubility of a solid in water can vary depending on factors such as temperature, pressure, and the presence of other solutes.
Learn more about solubility:
https://brainly.com/question/23946616
#SPJ1
What is the molar mass of this compound? XeF4

Answers
XeF⁴ has a mοlar mass οf abοut 207.2886 g/mοl.
What is mοlar mass?One mοle οf a material weighs οne mοlar mass. It is cοmputed by cοmbining the atοmic masses οf all the atοms in a mοlecule and is οften repοrted in grams per mοle (g/mοl). The mass οf an atοm in a chemical element is knοwn as its atοmic mass.
Hοw dο yοu determine it?The atοmic masses οf the cοmpοnents, which are: can be added tο determine the mοlar mass οf XeF⁴ (xenοn tetrafluοride).
Atοmic mass οf xenοn (Xe) is 131.293 g/mοl.
Atοmic mass οf fluοrine (F) is 18.9984 g/mοl.
As a result, the fοrmula fοr calculating the mοlar mass οf XeF⁴ is:
Mοlar mass οf XeF⁴ = (1 x mοlar mass οf Xe) + (4 x mοlar mass οf F) = (1x 131.293 g/mοl) + (4 x 18.9984 g/mοl) = 207.2886 g/mοl (apprοx)
To know more about molar mass, visit:
brainly.com/question/13152455
#SPJ1
When olive oil and sodium hydroxide are mixed together, soap and glycerol are made. The solubility of each of the materials are shown in the table below.
Material olive oil sodium hydroxide soap glycerol
Solubility not soluble very soluble soluble soluble
What is the best conclusion, based on the information given?
A.
This is not a chemical reaction because all of the materials mixed can be dissolved in water, and dissolving is a physical change.
B.
This is not a chemical reaction because all of the materials are liquids and can be completely dissolved in water.
C.
This is a chemical reaction because the materials that were mixed have different solubilities than the materials that were made.
D.
This is a chemical reaction because all of the materials used can be combined with water to produce a mixture.
Answers
Answer:
C
Explanation:
This is a chemical reaction because the materials that were mixed have different solubilities than the materials that were made.
Answer:
C
Explanation:
When a chemical reaction occurs, new materials form. Different materials each have unique physical properties, such as solubility.
When olive oil and sodium hydroxide were mixed together, soap and glycerol were made. The table shows that olive oil and sodium hydroxide have solubilities that are different than soap and glycerol. This means that olive oil and sodium hydroxide are different materials than soap and glycerol. Therefore, this is a chemical reaction because the materials that were mixed have different solubilities than the materials that were made.
Which of these is a pair of coordination isomers (aka ionization isomers)?
a. Na2[NiBr2Cl2] and K2[NiBr2Cl2]
b. [Ni(NH3)3Br]Cl and [Ni(NH3)3Cl]Br
c. [Ni(NH3)3(H2O)]SO4 and [Ni(NH3)2(H2O)2]SO4
Which of these pairs are linkage isomers??
a. [Pt(Cl)2(SCN)4]^4- and [Pt(Cl)2(NCS)4]^4-
b. [Pt(Cl)2(SCN)4]^4- and [Pt(Cl)4(SCN)2]^4-
c. K4[Pt(Cl)2(SCN)4] and Na4[Pt(Cl)2(SCN)4]
Answers
Answer: \([Ni(NH_3)_3Br]Cl\) and \([Ni(NH_3)_3Cl]Br\) are ionization isomers.
\([Pt(Cl)_2(SCN)_4]^{4-}\) and \([Pt(Cl)_2(NCS)_4]^{4-}\) are linkage isomers.
Explanation:
Ionization isomerism occur when a ligand that is bound to the metal center exchanges places with an anion or neutral molecule that was originally outside the coordination complex
Thus \([Ni(NH_3)_3Br]Cl\) and \([Ni(NH_3)_3Cl]Br\) are ionization isomers.
Linkage isomerism is the existence of coordination compounds that have the same composition differing with the connectivity of the metal to a ligand.
Thus \([Pt(Cl)_2(SCN)_4]^{4-}\) and \([Pt(Cl)_2(NCS)_4]^{4-}\) are linkage isomers.
What are some real-world examples of ''Convection''
please add at least 3 simple sentences
Answers
Some real-world examples of convection are as follows:
Boiling water: In this process, the heat from the burner gets transferred to the pot. Sea breeze: The formation of the sea and land breeze are the most potent examples of convection. Blood circulation in warm-blooded animals also involves the process of transfer of heat. What do you mean by Convection?Convection may be defined as the methodology through which heat is significantly transferred by the normal movement of a heated fluid such as air or water within the component.
The process of convection remarkably involves the transfer of heat through a fluid (liquid or gas) that is provoked by molecular motion.
Therefore, some real-world examples of convection are well-described above.
To learn more about Convection, refer to the link:
https://brainly.com/question/9382711
#SPJ1
mc016-1
Which represents the correct mass-volume relationship at STP?
32.00 g of O2 react with an excess of H2 to produce (2 ´ 22.4) L of H2O.
2.00 g of H2 react with an excess of O2 to produce (2 ´ 22.4) L of H2O.
32.00 g of O2 react with an excess of H2 to produce 22.4 L of H2O.
(2 ´ 2.00) g of H2 react with an excess of O2 to produce 22.4 L of H2O.
Answers
The correct mass-volume relationship at STP is A. 32.00 g of \(O_{2}\) react with an excess of \(H_{2}\) to produce (2 ´ 22.4) L of \(H_{2}O\)
To determine the correct mass-volume relationship at STP (Standard Temperature and Pressure), we need to consider the stoichiometry of the balanced chemical equation, the molar masses of the substances involved, and the molar volume of gases at STP.
The balanced equation indicates that 2 moles of \(H_{2}\) react with 1 mole of \(O_{2}\) to produce 2 moles of \(H_{2}O\). At STP, 1 mole of any gas occupies 22.4 liters.
In option A, it states that 32.00 g of \(O_{2}\) react with an excess of \(H_{2}\) to produce (2 × 22.4) L of \(H_{2}O\). The molar mass of \(O_{2}\) is 32.00 g/mol, which corresponds to 1 mole of \(O_{2}\). According to the balanced equation, 1 mole of \(O_{2}\) reacts to form 2 moles of \(H_{2}O\). Therefore, the given mass of \(O_{2}\) (32.00 g) corresponds to 1 mole of \(O_{2}\), which will produce 2 moles of \(H_{2}O\).
Since 1 mole of any gas occupies 22.4 L at STP, 2 moles of \(H_{2}O\) would occupy (2 × 22.4) L, which is equal to 44.8 L. Hence, the correct answer is A, where 32.00 g of \(O_{2}\) react with an excess of \(H_{2}\) to produce (2 × 22.4) L of \(H_{2}O\). This choice represents the correct mass-volume relationship at STP based on the given equation and stoichiometry. Therefore, Option A is correct.
The question was incomplete. find the full content below:
Consider the equation. 2\(H_{2}\)(g) + \(O_{2}\)(g) >>>> 2\(H_{2}O\)(g)
Which represents the correct mass-volume relationship at STP?
A. 32.00 g of \(O_{2}\) react with an excess of \(H_{2}\) to produce (2 ´ 22.4) L of \(H_{2}O\)B. 2.00 g of \(H_{2}\) react with an excess of \(O_{2}\) to produce (2 ´ 22.4) L of \(H_{2}O\).
C. 32.00 g of \(O_{2}\) react with an excess of \(H_{2}\) to produce 22.4 L of \(H_{2}O\)
D. (2 ´ 2.00) g of \(H_{2}\) react with an excess of \(O_{2}\) to produce 22.4 L of \(H_{2}O\).
Know more about molar volume here:
https://brainly.com/question/11676583
#SPJ8
Consider the following equilibrium:
N2(g) + 3H2(g) ⇌ 2NH3(g) + 92 kJ
The forward reaction is
Select one:
a.
exothermic and entropy is increasing.
b.
exothermic and entropy is decreasing.
c.
endothermic and entropy is constant.
d.
endothermic and entropy is increasing.
e.
endothermic and entropy is decreasing.
Answers
Answer:
b.exothermic and entropy is decreasing
What is the chemical formula for the ionic compound formed by Na+ and N¯³?
Answers
Answer:
Na3N
Explanation:
cuzdoodlikeimsosure
Can someone please help me with this? :(

Answers
Answer:
Bo
Explanation:
Bo is not a element