The ___ is the number of protons in the nucleus of an atom of an element.
Fill in the blank.
Answers
Related Questions
in the following equation, what hybridization change, if any, occurs for phosphorus? pcl3 cl2 → pcl5 no change sp2 → sp3 sp → sp2 sp3 → sp sp2 → sp3d sp3 → sp3d
Answers
The hybridization change that occurs for phosphorus in the reaction PCl3 + Cl2 → PCl5 is sp3 → sp3d.The hybridization change that occurs for phosphorus in the reaction PCl3 + Cl2 → PCl5 is sp3 → sp3d.PCl3 + Cl2 → PCl5The above reaction is a balanced chemical equation.
The phosphorus (P) atom in PCl3 has a hybridization of sp3, whereas the Cl2 molecule has a hybridization of sp2. During the formation of PCl5, there is a hybridization change in the phosphorus atom from sp3 to sp3d.A change in the hybridization of an atom occurs when it is involved in a chemical reaction. The changes in hybridization occur due to the difference in electronegativity of the atoms in the reactants or due to the bond formation.
For instance, in the reaction PCl3 + Cl2 → PCl5, the reaction occurs due to the formation of a covalent bond between the P atom in PCl3 and Cl atom in Cl2. This causes the electrons in the 3p subshell of the P atom to undergo excitation, resulting in the hybridization change from sp3 to sp3d.Hence, the correct answer is sp3 → sp3d.
To know more about chemical equation visit:-
https://brainly.com/question/29762834
#SPJ11
Similar plant and animal fossils
have been found on continents
that are now separated by an
ocean. Why is this significant?

Answers
A cell in your adrenal gland has about 2. 5 * 10^4 tiny compartments called vesicles that contain the hormone epinephrine (also called adrenaline). (a) An entire cell has about 150 fmol of epinephrine. How many attomoles (amol) of epinephrine are in each vesicle?
(b) How many molecules of epinephrine are in each vesicle?
(c) The volume of a sphere of radius r is r/3 πr^3. Find the volume of a spherical vesicle of radius 200 nm. Express your answer in cubic meters (m3 ) and liters, remembering that 1 L = 10^-3 m^3.
(d) Find the molar concentration of epinephrine in the vesicle if it contains 10 amol of epinephrine.
Answers
The values of all sub-parts have been obtained.
(a) The 6.04 amol/vescile of epinephrine are in each vesicle.
(b) The 3637892 molecules of epinephrine are in each vesicle.
(c) The volume of a spherical vesicle is V = 3.34 × 10⁻¹⁷ L.
(d) The concentration of epinephrine = 0.30 M.
What is molar concentration.
A chemical species' concentration, specifically the amount of a solute per unit volume of solution, is measured by its molar concentration. The number of moles per litre, denoted by the unit sign mol/L or mol/dm3 in SI units, is the most often used molarity unit in chemistry.
(a) Evaluate that how many attomoles (amol) of epinephrine are in each vesicle?
As given,
1 fmol = 10⁻¹⁵ mol
1 amol = 10⁻¹⁸ mol
Number of attomoles epinephrine:
= 151 fmol × (10⁻¹⁵ mol)/ 1 fmol × 1amol/10⁻¹⁸ mol
= 151000 amol
Number of attomoles of epinephrine in each vescile:
= 151000 amol/2.5 × 10⁴ vescile
= 6.04 amol/vescile.
(b) Evaluate that how many molecules of epinephrine are in each vesicle?
Number of molecules present in 1 mol of epinephrine:
= 6.04/vescile × [(10⁻¹⁵ mol)/ 1 fmol] × [6.023 × 10²³ molecules/1 mol]
= 3637892
(c) Evaluate the volume of a spherical vesicle of radius 200 nm.
Radius of spherical vescile is 2.00 × 10⁻⁷ m
Volume of the spherical vescile is,
V = 4/3 πr³
Substitute value of r respectively,
V = 4/3 π(2.00 × 10⁻⁷ )³
V = 3.34 × 10⁻²⁰ m³
V = 3.34 × 10⁻¹⁷ L
(d) Evaluate the molar concentration of epinephrine in the vesicle if it contains 10 amol of epinephrine.
Number of moles epinephrine = 10 amol
= 1.00 × 10⁻¹⁷mol
Volume of the spherical vescile = 3.34 × 10⁻¹⁷ L
Concentration of epinephrine = (1.00 × 10⁻¹⁷mol)/(3.34 × 10⁻¹⁷ L)
Concentration of epinephrine = 0.30 M
Hence, the values of all sub-parts have been obtained.
To learn more about Concentration of epinephrine from the given link.
https://brainly.com/question/13243272
#SPJ4
How many electrons are gained in the half-reaction 02, + electrons — 202-
Answers
Answer:
2
Explanation:
A
Answer: 4!!!
Explanation: j took the quiz!!!
What is kinetic energy
Answers
Kinetic energy is the energy of an object that it has because of it's movement. For example: That windmill has kinetic energy.
In SI Units, kinetic energy is measured in joules. Hope this helps!
Nitrogen gas is sealed in the container at STP. If the temperature inside the container doubled, the pressure will:
Answers
Sorry for no answer
What are the eight large, round bodies in the solar system that orbit, or revolve, around the sun and are usually ordered according to their distances from the sun?
Asteroids
Galaxies
Moons
Planets
Answers
Answer: D: planets
Explanation:
Why is a pyramid shape used to represent the energy in an Ecosystem?
Answers
Answer:
An energy pyramid shows the flow of energy at each trophic level in an ecosystem. A pyramid shape is used because energy is lost at each trophic level when organisms use it up.
Explanation:
How many moles of KOH are needed to neutralize 35.0 mL of 0.115 M HNO3 solution? А. 1.03 x 10^-3 moles B. 4.03 x 10^-1 moles C. 4.03 x 10^1 moles D. 4.03 x 10^-3 moles
Answers
(D) 4.03 x 10^-3 moles of KOH are needed to neutralize 35.0 mL of 0.115 M HNO3 solution.
The balanced chemical equation for the reaction between KOH and HNO3 is:
KOH + HNO3 → KNO3 + H2O
From the equation, we can see that 1 mole of KOH reacts with 1 mole of HNO3 to produce 1 mole of KNO3 and 1 mole of water. Therefore, we can use the following equation to calculate the moles of KOH needed to neutralize 35.0 mL of 0.115 M HNO3 solution:
moles of KOH = volume of HNO3 × concentration of HNO3
Before we substitute the values into the equation, we need to make sure that the volume of HNO3 is in liters, so we convert 35.0 mL to 0.0350 L. Then we substitute the values into the equation and get:
moles of KOH = 0.0350 L × 0.115 mol/L = 0.00403 mol
Therefore, the moles of KOH needed to neutralize 35.0 mL of 0.115 M HNO3 solution is 0.00403 mol, which is approximately equal to 4.03 x 10^-3 moles.
Know more about moles here: https://brainly.com/question/26416088
#SPJ4
Calcium has a density of 2.72 g/ml. What is the mass of 24.9 ml of calcium?
Answers
4 Elliot has some stearic acid. It is in the solid state.
a Describe how Elliot could use the apparatus below to measure the melting point of his
stearic acid.
Answers
Answer:
it is in solid state
Explanation:
at 100% heat
At regular intervals, check the stearic acid's temperature. Make a note of the findings and create a temperature vs time graph.
What is Temperature?The physical concept of temperature indicates in numerical form how hot or cold something is. A thermometer is used to determine temperature. Thermometers are calibrated using a variety of temperature scales, which historically defined distinct reference points and thermometric substances.
For above given example, check the temperature of the stearic acid often and make a note of the results and plot the temperature against time.
Temperature is not the same as the energy of a thermodynamic system; for instance, an iceberg has a significantly larger total heat energy than a match, despite the fact that a match is burning at a much higher temperature.
Thus, at regular intervals, check the stearic acid's temperature. Make a note of the findings and create a temperature vs time graph.
Learn more about Temperature, here:
https://brainly.com/question/7510619
#SPJ3
What properties do atoms of the same element share? Select all that apply
atomic number
chemical reactivity
melting point
Answers
Answer: your answer is A
Explanation: the atoms has the same properties of the same element as the atomic number.
Which represents a balanced nuclear equation?
1) 23/11Na ——>24/11Mg+1/1H
2) 24/11Na ——>24/12Mg+0/-1e
3) 24/13Al ——>24/12Mg+0/-1e
4) 23/12Mg ——>24/12Mg+1/0n
Answers
Answer:
The correct option is 2.
Explanation:
In a nuclear reaction balanced we have that:
1. The sum of the mass number (A) of the reactants (r) is equal to the sum of the mass number of the products (p) \( \Sigma A_{r} = \Sigma A_{p} \)
2. The sum of the atomic number (Z) of the reactants is also equal to the sum of the atomic number of the products \(\Sigma Z_{r} = \Sigma A_{p}\)
So, let's evaluate each option.
1) \(^{23}_{11}Na \rightarrow ^{24}_{11}Mg + ^{1}_{1}H\)
The mass number of the reactant is:
\(A_{r} = 23 \)
The sum of the mass number of the products is:
\( A_{p} = 24 + 1 = 25 \)
This is not the correct option because it does not meet the first condition (\( \Sigma A_{r} = \Sigma A_{p}\)).
2) \(^{24}_{11}Na \rightarrow ^{24}_{12}Mg + ^{0}_{-1}e\)
The mass number of the reactant and the products is:
\(A_{r} = 24 \)
\( A_{p} = 24 + 0 = 24 \)
Now, the atomic number of the reactants and the products are:
\(Z_{r} = 11 \)
\( Z_{p} = 12 + (-1) = 11 \)
This nuclear reaction is balanced since it does meet the two conditions for a balanced nuclear equation, (\( \Sigma A_{r} = \Sigma A_{p}\) and \( \Sigma Z_{r} = \Sigma Z_{p}\)).
3) \(^{24}_{13}Al \rightarrow ^{24}_{12}Mg + ^{0}_{-1}e\)
The mass number of the reactant and the products is:
\(A_{r} = 24 \)
\( A_{p} = 24 + 0 = 24 \)
Now, the atomic number of the reactants and the products are:
\(Z_{r} = 13 \)
\( Z_{p} = 12 + (-1) = 11 \)
This reaction does not meet the second condition (\( \Sigma Z_{r} = \Sigma Z_{p}\)) so this is not a balanced nuclear equation.
4) \(^{23}_{12}Mg \rightarrow ^{24}_{12}Mg + ^{1}_{0}n\)
The mass number of the reactant and the products is:
\(A_{r} = 23 \)
\( A_{p} = 24 + 1 = 25 \)
This reaction is not a balanced nuclear equation since it does not meet the first condition (\( \Sigma A_{r} = \Sigma A_{p}\)).
Therefore, the correct option is 2.
I hope it helps you!
As soon as you realize that you are in danger, you feel your heart speed up. You feel very aware of your surroundings. Your whole body is preparing to run or fight for your life. Which of the following would be the response of your nervous system?
A)heart rate speeds up
B)body moves to a safer place
C)senses become sharper so you are better able to respond
D)stress hormones are transported around the
Answers
Answer:
C would be the nervous system reacting
If you wanted to know about the other answers:
A and D are the circulatory system since it's transporting stress hormones through the blood and the heart rate speeds up
B is the muscular and skeletal system since they are the ones that make your body move
Question 5 of 10
What kind of land feature is shown at point on this topographic map?
A. Alake
B. A gentle slope
C. A mountaintop
D. A steep slope

Answers
Answer:A
Explanation:
gentle slope
what is the concentration of hydroxide ion in a 0.21 m aqueous solution of hydroxylamine, nh2oh? what is the ph?
Answers
The concentration of hydroxide ion in a 0.21 m aqueous solution of hydroxylamine, NH2OH, is 1.4 x 10^-6 M. The pH of the solution is 8.85.
Hydroxylamine, NH₂OH, is a weak base that can react with water to produce hydroxide ions, OH-. The equation for the reaction is:
NH₂OH, + H₂O ⇌ NH₃OH+ + OH-
The equilibrium constant for this reaction is Kb = 1.1 x 10^-8. To find the concentration of hydroxide ions, we can use the expression for Kb:
Kb = [NH₃OH+][OH-]/[NH₂OH,]
Since the concentration of hydroxylamine is 0.21 M, we can assume that the concentration of NH₃OH+ is negligible compared to NH₂OH. Therefore, we can simplify the expression to:
Kb = [OH-]^2/0.21
Solving for [OH-], we get:
[OH-] = sqrt(Kb x 0.21) = 1.4 x 10^-6 M
To find the pH of the solution, we can use the expression:
pH = 14 - pOH
pOH = -log[OH-] = -log(1.4 x 10^-6) = 5.85
pH = 14 - 5.85 = 8.85
Therefore, the concentration of hydroxide ion in a 0.21 m aqueous solution of hydroxylamine is 1.4 x 10^-6 M and the pH is 8.85.
Learn more about hydroxylamine
https://brainly.com/question/22564848
#SPJ11
which gas is prepared by heating ammonium chloride with calcium oxide?
Answers
Answer: The correct answer is Ammonia gas is prepared by heating ammonium chloride with calcium oxide
Hope this helps!
What is a foram and what is curious about the foram fossils of gubbio’s limestone?
Answers
Their fossilized shells formed these limestone rocks, which, like Gubbio's mountains, were once at the bottom of the ocean. Now exposed, they represent over a million years of a geological time period known as the Cretaceous.
Walter Alvarez found that forming a distinct boundary between the limestone of the two periods was a thin layer of red clay. Immediately below this clay boundary, the Cretaceous limestone was heavily populated with a wide mix of the fossils of tiny marine creatures called foraminifera, or “foram” for short.
On the Atlantic Coast, outside the town of Zumaia, Dutch geologist Jan Smit was studying the forams from a different ancient sea. Their fossilized shells formed these limestone rocks, which, like Gubbio's mountains, were once at the bottom of the ocean.
Learn more about limestone here: https://brainly.com/question/13723417
#SPJ4
The percent of heat radiation reflected by the different types of surfaces that earth has (i.e. snow, rock, forest, etc.) can be quantified or measured as?
Answers
The percent of heat radiation reflected by the different types of surfaces that Earth has (i.e snow, rock, forest, etc.) can be quantified or measured as Albedo.
Albedo is the percentage of sunlight that is reflected off of a surface. The higher the albedo, the more reflective the surface is. Different surfaces have different albedos. For example, fresh snow has a high albedo, while a forest has a low albedo.
The albedo of a surface can have a big impact on the climate. If a surface has a high albedo, it will reflect more heat and stay cooler. If a surface has a low albedo, it will absorb more heat and become warmer.
You can measure the albedo of a surface with a simple device called an albedometer. By measuring the amount of sunlight that is reflected off of a surface, you can calculate the albedo.
Surfaces with a high albedo are important in helping to regulate the Earth’s climate. They help keep the Earth cool by reflecting back sunlight.
Learn more on albedo here:
https://brainly.com/question/1335260
#SPJ4
What is the electron configuration of a fluoride ion (F-) in the ground state?
1522s22p?
1822s22p4
1522s22p6
1522s22p5
Answers
What mass of iodine, I2 (molar mass 253.80 g/mol), must be used to prepare a 0.960 m solution if 100.0 g of ethanol, C2H5OH, is used
Answers
To prepare a 0.960 m solution using 100.0 g of ethanol, we need 243.65 g of iodine.
To determine the mass of iodine needed to prepare a 0.960 m solution using 100.0 g of ethanol, we first need to calculate the number of moles of ethanol present:
moles of ethanol = mass of ethanol / molar mass of ethanol
moles of ethanol = 100.0 g / 46.07 g/mol
moles of ethanol = 2.17 mol
Next, we can use the molarity equation to calculate the number of moles of iodine needed for the solution:
molarity = moles of solute / liters of solution
Since we don't know the volume of the solution, we can assume it is 1 liter to make the calculation easier. Therefore:0.960 m = moles of iodine / 1 L
moles of iodine = 0.960 mol
Finally, we can use the number of moles of iodine needed to calculate the mass of iodine required:
mass of iodine = moles of iodine x molar mass of iodine
mass of iodine = 0.960 mol x 253.80 g/mol
mass of iodine = 243.65 g
Learn more about solution: https://brainly.com/question/30665317
#SPJ11
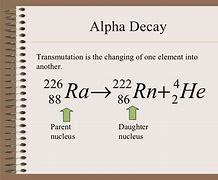
226 • Ra 4/2 He + 88 = ?
Answers
Answer: the answer for this question is in the pic
Explanation:
Matter takes up _____.
(a) Space and temperature
(b) Space and heat
(c) Space and mass
(d) Heat and temperature
Answers
Select the substance(s) that confirm the presence of Pb2+ ions.
a. Ammonia followed by hydrochloric acid
b. None of these
c. Ammonia followed by potassium ferrocyanide
d. Sodium iodide
e. Potassium thiocyanate
Answers
To confirm the presence of \(\(Pb^2^+\)\) ions, the substance that can be used is potassium thiocyanate (KSCN). Therefore, the correct answer is: e. Potassium thiocyanate
When potassium thiocyanate is added to a solution containing \(Pb^2^+\) ions, it forms a reddish-brown precipitate called lead(II) thiocyanate \((Pb(SCN)^2)\). This reaction is a common test for the presence of \(Pb^2^+\) ions.
The reaction can be represented by the following equation:
\(Pb^2^+\) (aq) + 2 \(SCN^-[/tex} (aq) → \(Pb(SCN)^2\) (s)
In this reaction, the Pb2+ ions from the solution react with the thiocyanate ions (SCN-) to form a reddish-brown precipitate of lead(II) thiocyanate. The formation of the precipitate confirms the presence of \(Pb^2^+\) ions in the solution.
To know more about Potassium Thiocyantae:
https://brainly.com/question/28300500
#SPJ11
What is the difference between a reflected and a diffracted sound wave?
Answers
Answer:
Diffraction involves a change in direction of waves as they pass through an opening or around a barrier in their path.
Reflection involves a change in direction of waves when they bounce off a barrier; refraction of waves involves a change in the direction of waves as they pass from one medium to another
Explanation:
If we use water waves as an example, waves hitting shallower water at an angle will slow down and change direction slightly. That is refraction
Waves hitting an island will bend and eventually close in on the "shadow" of the island. That is diffraction
I will give brainliest to first person who answers correctly.
What category is the single greatest source (Widespread Forest Fires, Biological Emissions/Cattle, Energy Production, Chemical Waste) of our carbon footprint, and where would we find the largest impact of this footprint?
Answers
Answer:
Energy Production and China
Explanation:
China is the biggest carbon footprint contributior due to the mass manufacturing and buildings they have.
Which of the following statements is true? A. Isotopes of the same element have the same number of protons and neutrons. B. Isotopes have the same physical properties as the normal atom but different chemical properties. C. Isotopes have the same chemical properties as the normal atom but different physical properties. D. If an isotope of one element has the same atomic mass as another element, they will have the same properties.
Answers
Answer:
B
Explanation:
Isotopes have the same number of protons but different number of neutrons.
The true statement is,
Isotopes have the same chemical properties as the normal atom but different physical properties.So, option C is correct one.
What is isotopes?The elements have same number of proton but different number of neutrons.Example: hydrogen, deuterium, tritiumWhy isotopes of same elements have different physical properties?The isotopes have different physical properties like freezing point , mass, melting or boiling point etc because they have different mass number .
learn about isotopes,
https://brainly.com/question/11904263
#SPJ2
what is the physical state of oxygen at room temperature
Answers
The psychical state of oxygen at room temperature is gas.
Explanation:
Oxygen has low melting and boiling points, so it is in a gas state at room temperature.
the following two reactions are important in the blast furnace production of iron metal from iron ore (fe2o3): using these balanced reactions, how many moles of o2 are required for the production of 3.36 kg of fe?
Answers
In the blast furnace production of iron metal from iron ore 45 moles of O₂ will be required for the production of 3.36 kg of iron metal.
In the blast furnace production of iron metal from iron ore (Fe₂O₃), the two important reactions are:
1. Fe₂O₃ + 3CO → 2Fe + 3CO₂
2. Fe₂O₃ + 3C → 2Fe + 3CO
To determine how many moles of O2 are required for the production of 3.36 kg of iron metal, follow these steps:
1-Convert the mass of Fe to moles.
1 mole of Fe has a mass of approximately 56 g (using the atomic weight of iron).
Therefore, 3.36 kg = 3360 g
moles of Fe = 3360 g / 56 g/mol = 60 moles
2-Use the stoichiometry of the reactions to find moles of Fe₂O₃.
From the balanced reactions, 2 moles of Fe are produced from 1 mole of Fe₂O₃.
Therefore, moles of Fe₂O₃ = 60 moles Fe / 2 = 30 moles
3-Determine the moles of O₂ required.
1 mole of Fe₂O₃ contains 3 moles of O atoms.
Since O₂ is diatomic, 1 mole of O₂ contains 2 moles of O atoms.
Therefore, moles of O atoms in Fe₂O₃ = 30 moles
Fe2O3 * 3 = 90 moles
moles of O₂ required = 90 moles O / 2 = 45 moles O₂
In conclusion, 45 moles of O₂ are required for the production of 3.36 kg of iron metal.
Learn more about "Blast furnace production"; https://brainly.com/question/13484619
#SPJ11
A solution is prepared by adding 91.9 g of ethanol, ch3ch2oh, to 481.0 ml of water. the final volume of the solution is 597.5 ml. assuming the density of water is 1.0 g/ml and the density of ethanol is 0.789 g/ml, calculate the molality of the ethanol in the solution.
Answers
Answer:
17.51M
Explanation:
first we make out what we've been given .
Mass of ethanol= 91.9g
volume of water = 481.0ml
volume of solution= 597.5ml
density of water = 1.0g/ml
density of ethanol= 0.789g/ml.
firstly find the volume of ethanol since the total volume of the solution is 597.5ml and we've been given the volume of water which is 481.0ml , we subtract the volume of water from the volume of the solution , to find out the volume of ethanol .
volume e =597.5ml - 481.0ml
= 116.5ml
Then we find the molecular mass of ethanol , which is 45.04g/mol .
then we find the number of moles of ethanol .
n=mass /molar mass
=91.9g/ 45.04g/mol
=2.04mols .
Then convert the volume from milliliters to liters , by dividing by a 1000 .
v= 116.5/1000
=0.1165L
since we have our number of moles and volume , we can now find the molarity . which is
M=n/v
= 2.04mols /0.1165L
= 17.51M .