the hot box is usually set between what temperature range?
Answers
The hot box is typically set within a temperature range of 150 to 200 degrees Celsius.
The hot box is a controlled environment used in various industries, including food processing, laboratory testing, and material research. It is designed to maintain a specific temperature for a given duration. The temperature range for a hot box typically falls between 150 to 200 degrees Celsius. This range provides a significant degree of flexibility for different applications.
Setting the hot box within this temperature range allows for efficient heating and testing of various materials and products. It is crucial to consider the specific requirements of the process or experiment when determining the precise temperature within this range. Factors such as the nature of the materials being tested, desired reaction rates, and safety considerations play a role in determining the appropriate temperature setting.
By maintaining a consistent temperature within the specified range, the hot box ensures reliable and reproducible results. It provides a controlled environment for processes that require elevated temperatures, such as drying, curing, sterilization, or accelerated aging. The ability to set and maintain a specific temperature range is essential for achieving accurate and consistent outcomes in a wide range of industrial and scientific applications.
To learn more about temperature refer:
https://brainly.com/question/2339046
#SPJ11
Related Questions
what is 37degrees Celsius in Si units
Answers
Answer:
310.15 Degrees Kelvin
Explanation:
37 Degrees Celsius = 310.15 Degrees Kelvin = 98.6 Degrees Fahrenheit
Conversion Factor from Celsius to kelvin
or 37 + 273 = 310 Degrees Kelvin
Will give brainliest if correct
Based on what you have observed in the Gizmo, which of the following chemical equations represents photosynthesis?
A. Carbon dioxide plus water yields glucose (sugar) and oxygen.
B. Oxygen plus carbon dioxide yields water and glucose.
C. Glucose plus oxygen yields carbon dioxide and water.
D. Water plus glucose yields oxygen and carbon dioxide.
Answers
Answer:
A.....
Explanation:
Carbon dioxide plus water yields glucose (sugar) and oxygen.
A solution contains 37.5 grams of calcium carbonate (caco3) in 500 ml of
water. what is the concentration of this solution?
Answers
The concentration of the solution is 0.748 M.
To find the concentration of the solution, we need to calculate the number of moles of calcium carbonate present in the solution.
First, we need to determine the molecular weight of calcium carbonate (\(CaCO3\)).
\(CaCO3\) = 1 x Ca + 1 x C + 3 x O
= 40.08 g/mol + 12.01 g/mol + (3 x 16.00 g/mol)
= 100.09 g/mol
Next, we can use the formula:
concentration (in mol/L) = moles of solute / volume of solution (in L)
We have 37.5 grams of calcium carbonate in 500 ml of water. To convert ml to L, we divide by 1000:
volume of solution = 500 ml / 1000 ml/L = 0.5 L
moles of calcium carbonate = mass / molecular weight
= 37.5 g / 100.09 g/mol
= 0.374 moles
Therefore, the concentration of the solution is:
concentration = 0.374 moles / 0.5 L
= 0.748 M
The concentration of the solution is 0.748 M.
To know more about concentration refer to-
https://brainly.com/question/10725862
#SPJ11
In an experiment, 3.25 g of C3H8 react with 3.50 g of O2.1) Write the formula for the reactant that is the limiting reactant.
Answers
So,
The reaction that takes place here is the next one:
The first thing we're going to do is to pass the mass of each compound to moles. (We do this just dividing by the molecular weight of the compound):
Now, the last step is just to divide each amount by the coefficient of the reaction of each compound. The smaller result will be the limiting reactant.
Therefore, the limiting reactant is O2.
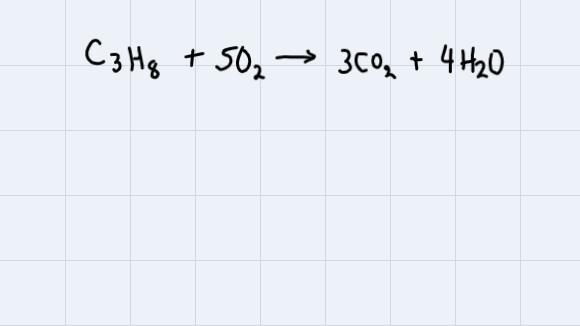


How many mols of H2SO4 is in 0.80 L of 1.40 M H2SO4?
Answers
g calculate the partial pressure of water in the earth's atmosphere if water is 20 c and the relative humidity is 50%
Answers
The partial pressure of water in the Earth's atmosphere at 20°C with a relative humidity of 50% is approximately 11.75 hPa.
To calculate the partial pressure of water vapor in the Earth's atmosphere, we can use the concept of vapor pressure and the relative humidity.
Find the saturation vapor pressure (Pₛₐₜ) of water at 20°C:
The saturation vapor pressure can be determined using empirical equations or reference tables. At 20°C, the saturation vapor pressure of water is approximately 23.5 millibars (mb) or 23.5 hPa.
Calculate the partial pressure of water vapor (Pᵥ) using the relative humidity (RH):
The relative humidity is the ratio of the actual vapor pressure (Pᵥ) to the saturation vapor pressure (Pₛₐₜ), expressed as a percentage. In this case, the relative humidity is 50%.
Pᵥ = (RH/100) * Pₛₐₜ
Pᵥ = (50/100) * 23.5 mb
Pᵥ = 11.75 mb
Therefore, the partial pressure of water vapor in the Earth's atmosphere at 20°C with a relative humidity of 50% is approximately 11.75 millibars (mb) or 11.75 hPa.
You can learn more about vapor pressure at
https://brainly.com/question/2693029
#SPJ11
Kim loại đòng sắt đc tạo từ nguyên tố nào
Answers
Answer:
sup brö how is it back home?
Explanation:
just curious you know
a solution of acetic acid that has a concentration of 0.10 moles per liter has a ph of 2.87. what is the likely ph of a 0.10 mole per liter solution of the conjugate base sodium acetate?
Answers
0.10 moles per liter solution of the conjugate base sodium acetate is likely to have a pH greater than 7.
Is the pH of a 0.10 mole per liter solution of the conjugate base sodium acetate likely to be acidic or basic?When acetic acid (CH3COOH) donates a proton, it forms its conjugate base, acetate ion (CH3COO-). In the given scenario, the acetic acid solution has a pH of 2.87, indicating acidity. The lower pH value suggests a higher concentration of H+ ions. As a weak acid, acetic acid partially dissociates, releasing H+ ions and acetate ions. When sodium acetate (CH3COONa) dissolves in water, it completely dissociates into sodium ions (Na+) and acetate ions. The presence of acetate ions (the conjugate base) from sodium acetate will react with the excess H+ ions in the solution, shifting the equilibrium towards the formation of acetic acid and water. This process, called the hydrolysis of salts, will consume the H+ ions, thereby increasing the pH of the solution. Consequently, the 0.10 mole per liter solution of sodium acetate is likely to have a pH greater than 7, making it basic.
Learn more about pH
brainly.com/question/2288405
#SPJ11
If the pressure of a gas sample is quadrupled and the absolute temperature is doubled, by what factor does the volume of the sample change
Answers
Answer:
The new volume of the sample is halved.
Explanation:
Data obtained from the question include the following:
Initial volume (V1) = V
Initial temperature (T1) = T
Initial pressure (P1) = P
Final pressure (P2) = quadrupled = 4P
Final temperature (T2) = doubled = 2T
Final volume (V2) =?
Thus, we can obtain the new volume of the same by using the combined gas equation as shown below:
P1V1 /T1 = P2V2 /T2
P × V/T = 4P × V2/2T
Cross multiply
T × 4P × V2 = P × V × 2T
Divide both side by T × 4P
V2 = (P × V × 2T) / (T × 4P)
V2 = V/2
V2 = ½V
Therefore, the new volume of the sample is halved .
Compound A solid
Compound B solid
Compound
C
Compound
D
B
C
gas
D
solid
808
1610
-56
680
yes
no
no
Compound B because it has a very high melting point.
yes
You are studying the differences between ionic and covalent compounds. After studying the properties of each
type of compound, your teacher has provided this table and asked you to (1) identify the ionic compound(s) and
explain your choice(s).
A
Compounds A and D because they have relatively high melting points.
AROLIChessies
Compounds A, B, and C because they are solid at room temperature.
Compounds A and D because the conduct electricity in aqueous solutions.
yes
no
yes
yes

Answers
The Compounds A, B, and C are ionic compounds because they are solid at room temperature. Therefore, option B is correct.
What are ionic compound ?A chemical compound known as an ionic compound is one that contains ions bound together by the electrostatic forces known as ionic bonding. Despite having both positively and negatively charged ions, or cations and anions, the molecule is generally neutral.
Ionic chemicals are brittle and rigid. When an ionic chemical is dissolved in water, it separates into ions. Ionic compound solutions and melting forms of these substances carry electricity, while solid materials do not. The chemical formula of an ionic compound is metal + nonmetal or polyatomic ions.
Thus, option B is correct.
To learn more about an ionic compound, follow the link;
https://brainly.com/question/9167977
#SPJ1
Select the correct answer.
Who pioneered the use of galvanoplastic compounds for preserving footprints and ballistics?
OA Mathieu Orfila
B. Calvin Goddard
Oc. Edmon Locard
OD. Alphonse Bertillon
O E. Dr. Joseph Bell
Answers
Biometrics was first applied to law enforcement by Alphonse Bertillon, a French police officer, and researcher who borrowed the anthropological method of anthropometry to develop a biometric identification system therefore option D. is the right choice.
The police department's use of anthropometry as a means of criminal identification was the first scientific method of its kind. In the past, authorities could only track down offenders using their names or photographs. Fingerprinting ultimately replaced the technique.
Furthermore, he created the first-ever mug shot. Before Bertillon formalized the practice in 1888, offenders had already been photographed as early as the 1840s, just a few years after the introduction of photography.
In the historic Dreyfus case, Alfred Dreyfus has wrongfully convicted thanks to his faulty evidence.
Want to know more about galvanoplastic compounds visit the link which is given below;
https://brainly.com/question/17824025
#SPJ4
the volume of 5.00 M KBr needed to have 1.00 mol KBr mL
Answers
Answer: molarity = mole solute/ liter of solution.
=> volume= mole/molarity = 1/5 = 0.2 L = 200mL
Explanation:
Question 4 of 5
Suppose a disease wipes out most of the fox population in
the food web shown.
Mountain
lion
Deer
Hawk
Fox
Rabbit
Owl
Snake
Green
plant
Mouse
Which two populations are most likely to increase as a
result?
Answers
Answer:
Rabbit and Mouse
Explanation:
The rabbit and mouse are the prey of foxes.
If you have 3.62 moles of Tin (Sn), how many grams do you have?
a
.031 grams
b
118.711 grams
c
32.79 grams
d
429.73 grams
Answers
Answer:
D.) 429.73 grams
Explanation:
number of moles x molar mass= mass in grams
3.62 x 118.71= 429.73 grams
the electrons in the space formed by the overlapping atomic orbitals could have the same spin true or false
Answers
The Pauli Exclusion Principle states that no two electrons in an atom can share the same set of quantum numbers. So, the given statement is False.
Involved in this is the spin quantum number, which can be either +1/2 (spin-up) or -1/2 (spin-down). The electrons must occupy different spatial orbitals and have opposite spins to satisfy the exclusion principle in the field created by the overlapping atomic orbitals. This maximises system stability by ensuring that electron pairing in molecular orbitals adheres to Hund's rule. Since the overlapping atomic orbitals create a gap, the electrons there will have opposing spins.
So, the given statement is False.
Learn more about quantum number, here:
https://brainly.com/question/32773003
#SPJ4
Does anyone know Bible thing cuz I don’t know what to write and I need help if you know this ok

Answers
I answered all of them except 2 for you to do
Hope this helps :))
When an atom becomes a negative ion, it
Answers
It becomes a positives atom. Since it loses electrons it has more of a positive charge than a normal atom.
what type of reaction is performed with the elephant toothpaste demonstration?
Answers
The reaction performed with the elephant toothpaste demonstration is known as a decomposition reaction.
Decomposition Reaction:The process of breaking down a chemical compound into smaller molecules, atoms, or ions is known as a decomposition reaction. It is also known as analysis or disintegration. A reaction in which a single substance is broken down into two or more simpler substances is known as a decomposition reaction. The elephant toothpaste demonstration is a simple chemical reaction in which hydrogen peroxide breaks down into oxygen gas and water in a matter of seconds.
The formula for hydrogen peroxide is H₂O₂. It is a pale blue liquid that contains hydrogen, oxygen, and water. When you add yeast, soap, and food coloring, the reaction is more exciting. The yeast acts as a catalyst, breaking down hydrogen peroxide into water and oxygen gas. The oxygen gas created causes the soap to foam up, creating the "elephant toothpaste" effect. The chemical reaction that takes place during the elephant toothpaste demonstration can be written as follows:
2H₂O₂(liquid) → 2H₂O (liquid) + O₂ (gas)
This reaction is an example of a decomposition reaction.
To know more about decomposition reaction visit:
https://brainly.com/question/32864042
#SPJ11
13. An organic compound is found to contain 77.42% of C, 7.53% of H and
nitrogen. The mass of 1.12L of its vapour at NTP is 4.65g. Determine
the
empirical and molecular formula of the compound.
Answers
Answer
7.53% 97% if you divide it you can get the answer
Explanation:
Use the drop-down menus to name these
structures.
cis-3-decene
cis-3-nonene
trans-3-decene
trans-3-nonene
Answers
Using drop-down menu , IUPAC name is as follows :
cis-3-decene: (Z)-3-decene , cis-3-nonenetriene: (Z,Z,Z)-3-nonenetriene
trans-3-decene: (E)-3-decene , trans-3-nonene: (E)-3-nonene
cis-3-decene is an alkene with a double bond between carbon atoms 3 and 4, and both alkyl groups (attached to the double bond) on the same side of the double bond. Therefore, its IUPAC name is (Z)-3-decene.
cis-3-nonenetriene is a triene with three double bonds. The double bonds are between carbon atoms 3 and 4, 6 and 7, and 9 and 10. Since all the alkyl groups attached to the double bonds are on the same side of the double bonds, the compound is named as (Z,Z,Z)-3-nonenetriene. trans-3-decene is an alkene with a double bond between carbon atoms 3 and 4, and both alkyl groups (attached to the double bond) on opposite sides of the double bond. Therefore, its IUPAC name is (E)-3-decene. trans-3-nonene is an alkene with a double bond between carbon atoms 3 and 4, and both alkyl groups (attached to the double bond) on opposite sides of the double bond. Therefore, its IUPAC name is (E)-3-nonene.
For more such questions on IUPAC name
https://brainly.com/question/31569541
#SPJ8
A student determines the density of water obtained from a tap to be 1.015 g/mL. After measuring the mass and volume of the tap water the student filters it by distillation and reverse osmosis. The student then determines that the density of this filtered water is 0.993 g/mL. Calculate the percent error in both measurements assuming the true value to be 0.997 g/mL. What could cause these errors
Answers
The percent error in the measurement of the tap water density is approximately 1.02%, while the percent error in the measurement of the filtered water density is approximately -0.40%.
To calculate the percent error, we use the formula:
Percent Error = (|Measured Value - True Value| / True Value) x 100
For the measurement of the tap water density:
Percent Error = (|1.015 g/mL - 0.997 g/mL| / 0.997 g/mL) x 100
Percent Error ≈ (0.018 g/mL / 0.997 g/mL) x 100
Percent Error ≈ 1.81%
For the measurement of the filtered water density:
Percent Error = (|0.993 g/mL - 0.997 g/mL| / 0.997 g/mL) x 100
Percent Error ≈ (0.004 g/mL / 0.997 g/mL) x 100
Percent Error ≈ 0.40%
The positive percent error in the tap water measurement indicates that the measured value is higher than the true value. This could be due to experimental errors such as inaccuracies in the measuring equipment, variations in temperature affecting the density, or impurities present in the tap water.
The negative percent error in the filtered water measurement indicates that the measured value is lower than the true value. This could be caused by the loss of dissolved substances during the filtration process or inaccuracies in the measuring equipment.
The tap water density measurement has a percent error of approximately 1.02%, indicating a slight overestimation. The filtered water density measurement has a percent error of approximately -0.40%, indicating a slight underestimation. Possible causes for these errors include experimental inaccuracies, variations in temperature, impurities in the tap water, and losses of dissolved substances during filtration.
To know more about density visit:
https://brainly.com/question/28348989
#SPJ11
what does new substances often have that are different from the reactants
Answers
Answer
The new substances often have different combinations of atoms different from the reactants.
Explanation
The reactants and the new substances in a chemical reaction contain the same atoms, but they are rearranged during the reaction. As a result, the atoms end up in different combinations in the new substances. This makes the products new substances that are chemically different from the reactants.
How many grams of lithium is needed to react with 12.6L of nitrogen gas measured at 745mmHg and 28.5C?
Answers
Answer:
2.976 mol * 6.94 g/mol of lithium is needed to react with 12.6 L of nitrogen gas.
Explanation:
To determine the amount of lithium (Li) needed to react with a given volume of nitrogen gas (N2), we need to consider the balanced chemical equation and the ideal gas law.
What is the ideal gas law?
The ideal gas law is a fundamental equation in thermodynamics that describes the behavior of an ideal gas. It relates the pressure (P), volume (V), temperature (T), and number of moles (n) of a gas using the following equation:
PV = nRT
According to the given question:
Volume of nitrogen gas (V) = 12.6 L
Pressure of nitrogen gas (P) = 745 mmHg
Temperature of nitrogen gas (T) = 28.5 °C
We'll follow these steps to calculate the amount of lithium required:
Step 1: Convert the temperature to Kelvin. T(K) = T(°C) + 273.15 T(K) = 28.5 °C + 273.15 T(K) ≈ 301.65 K
Step 2: Convert the pressure from mmHg to atm. Pressure (P) = 745 mmHg / 760 mmHg/atm Pressure (P) ≈ 0.979 atm
Step 3: Apply the ideal gas law equation to calculate the number of moles of nitrogen gas (N2). PV = nRT
n = PV / RT
R = 0.0821 L•atm/(mol•K) (ideal gas constant)
n = (0.979 atm) * (12.6 L) / (0.0821 L•atm/(mol•K) * 301.65 K) n ≈ 0.496 mol
Step 4: Use the balanced chemical equation to determine the stoichiometry between lithium (Li) and nitrogen gas (N2). The balanced equation is: 6Li + N2 → 2Li3N
From the equation, we can see that 6 moles of lithium react with 1 mole of nitrogen gas.
Step 5: Calculate the moles of lithium needed based on the stoichiometry. moles of Li = (6 moles Li / 1 mole N2) * 0.496 mol N2 moles of Li ≈ 2.976 mol
Step 6: Calculate the mass of lithium using the molar mass of lithium.
Molar mass of Li = 6.94 g/mol mass of Li = moles of Li * molar mass of Li
Mass of Li ≈ 2.976 mol * 6.94 g/mol
The calculated mass of lithium will give us the amount of lithium needed to react with 12.6 L of nitrogen gas at the given conditions.
To know more about ideal gas law, refer here:
https://brainly.com/question/27870704
#SPJ4
What is the best Dog Training Organization for German Shepherds
Answers
Answer:
see it at Google search it
Answer:
The Police Station
Explanation:
They discipline the dogs to be trained
What is the reaction classification of the above reaction? A2B (l) → A2 (g) + B2 (g)
Answers
The reaction classification of the reaction, \(A_2B (l) -- > A_2 (g) + B_2 (g)\), is a decomposition reaction.
What is a decomposition reaction?In chemistry, a decomposition reaction is one in which a compound or a reactant decomposes to produce two or more chemically unique substances as the products.
Thus, a typical decomposition reaction can be written as:
AB ---> A + B
Decomposition reactions are the opposite of combination reactions. In combination reactions, two or more reactants combine to form a single product. A combination reaction can be written as:
A + B ---> AB
Considering the reaction in question: \(A_2B (l) -- > A_2 (g) + B_2 (g)\)
\(A_2B\) is the reactant. It undergoes a reaction to produce \(A_2\) and \(B_2\) respectively. In other words, the reaction is a decomposition reaction.
More on decomposition reactions can be found here: https://brainly.com/question/8009068
#SPJ1
what systems might not work right when you have a cold
Answers
Answer:
hello :3
A cold is a contagious upper respiratory infection that affects your nose, throat, sinuses and trachea (windpipe).
Explanation:
have a nice day :3
What does a percent recovery of more than 100% likely indicate?.
Answers
When the percent recovery is more than 100%, it means that you have more of the desired compound than you started with. The percent recovery is a measure of the amount of a desired substance that is obtained from a reaction, and it is typically calculated as follows: percent recovery = (actual yield / theoretical yield) x 100%If the percent recovery is more than 100%, it means that the actual yield is greater than the theoretical yield.
This can occur for a variety of reasons, such as: Contamination: The product may be contaminated with other substances that increase the mass of the sample. Reaction side products: A reaction may produce more than one product, and some of the byproducts may be included in the mass measurement.
Analytical error: There may be an error in the measurement of the mass of the sample or the product. These errors can occur due to a variety of factors, such as instrument calibration, sample preparation, or human error. In conclusion, a percent recovery of more than 100% likely indicates that you have more of the desired compound than you started with, but it is important to investigate the reasons for this result to ensure that it is accurate and reproducible.
To know more about recovery visit:
https://brainly.com/question/1528638
#SPJ11
Find the mass in kilograms in 2.6 mol of C8H12 (which is called windowpane).
Answers
Considering the definition of molar mass, the mass in 2.6 moles of C₈H₁₂ is 0.2808 kg.
Definition of molar massThe molar mass of an atom or molecule is the mass of one mole of that particle expressed in grams.
The molar mass of a compound is the sum of the molar mass of the elements that form it multiplied by the number of times they appear in the compound.
Mass of 2.6 mol C₈H₁₂Being the molar mass of C₈H₁₂ 108 g/mole, you can apply the following rule of three: If by definition of molar mass 1 mole of the compound contains 108 grams, 2.6 moles of the compound contains how much mass?
mass= (2.6 moles× 108 grams)÷ 1 mole
mass= 280.8 grams= 0.2808 kg (being 1000 gr= 1 kg)
Finally, the mass is 0.2808 kg.
Learn more about molar mass:
brainly.com/question/5216907
brainly.com/question/11209783
brainly.com/question/7132033
brainly.com/question/17249726
#SPJ1
The difference between a mixture and a solution is that mixtures cannot be easily separated. True O False.
Answers
Answer:
False
Explanation:
Mixtures are a solute and solvent that are not chemically combined, therefore easily separated. Solutions are a solute and solvent combined together.
as a system reaches thermal equilibrium, _____ has been transferred from areas of high to low _____.
Answers
As a system reaches thermal equilibrium, heat has been transferred from areas of high to low temperature.
In other words, when a system reaches thermal equilibrium, there is no longer a temperature difference between its different parts, and the heat has been evenly distributed throughout the system. This can happen through a variety of mechanisms, such as conduction, convection, and radiation, and the specific mechanism will depend on the properties of the system and the materials it is made of. Once thermal equilibrium has been reached, the system will remain at a constant temperature until something changes, such as the addition or removal of heat or a change in the materials or structure of the system.
to know more about heat-
https://brainly.com/question/13860901
#SPJ4