The criterion of -850mV is referenced to which electrode?
A) Calomel
B) CSE
C) silver-silver chloride
D) Zinc
Answers
The criterion of -850mV is referenced to the silver-silver chloride electrode (Ag/AgCl). Therefore the correct option is option C.
In electrochemistry, the silver-silver chloride electrode is frequently used as a standard reference electrode in studies of corrosion and other electrochemical reactions.
The potential of the silver-silver chloride electrode, which is defined at 0.1976 V vs the standard hydrogen electrode (SHE) at 25°C, is stable and repeatable.
In investigations on corrosion, the corrosion potential—the potential at which the rate of corrosion is minimized—is frequently determined using the criterion of -850 mV.
The corrosion potential is normally evaluated in relation to the silver-silver chloride electrode, and a corrosion study's standard criterion is typically a potential of -850 mV versus Ag/AgCl. Therefore the correct option is option C.
For such more question on electrode:
https://brainly.com/question/28302450
#SPJ11
Related Questions
In what part of this system are the two energy outputs found?

Answers
The part of the system that has two outputs of energy is the bulb.
What is the energy transformation?We would have to recall that on the basis of the first law of thermodynamics, it can be said that energy can not be created nor destroyed but that energy would have to be converted from one form to the other.
In this case, we can see that as the boy is cycling, there is the conversion of the mechanical energy of the boy to the heat and the light energy of the bulb as shown. These are the outputs referred to above.
Learn more about energy:https://brainly.com/question/1932868
#SPJ1
50.0 ml of 0.010m naoh was titrated with 0.50m hcl using a dropper pipet. if the average drop from the pipet has a volume of 0.040 ml, calculate how many drops of hcl are needed to reach the endpoint of the titration.
Answers
25 drops of acid is required to neutralize the 50.0 ml of 0.010m of NaOH in the experiment.
The equation of the reaction is;
NaOH(aq) + HCl(aq) ---------> NaCl(aq) + H2O(l)
We can use the titration formula;
CAVA/CBVB = NA/NB
CA= concentration of acid
VA = volume of acid
CB = concentration of base
VB = volume of base
NA = number of moles of acid
NB = number of moles of base
CB = 0.010 M
VB = 50.0 ml
CA = 0.50 M
VA = ?
NA = 1
NB = 1
Substituting values;
CAVANB = CBVBNA
VA = 0.010 × 50.0 × 1/ 0.50 × 1
VA = 1 ml
Since the total volume of acid used is 1 ml and each drop contains 0.040 ml
The number of drops required is 1ml/0.040 ml = 25 drops
Learn more: https://brainly.com/question/1527403
how does kinetic molecular theory explain gas pressure inside a balloon? select the correct answer below: gas particles are large and only a few fit inside a balloon, inflating the walls. the atmospheric pressure is greater than the pressure of the gas inside the balloon, so the balloon expands gas particles are very small, and many will fill the inside of the balloon until they are all stationary and touching each other. the pressure exerted by a gas in the balloon results from collisions between the gas molecules and the balloon walls.
Answers
Answer:
Therefore the answer is d) The pressure exerted by a gas in the balloon results from collisions between the gas molecules and the balloon walls.
Explanation:
2-Methyl-2-butanol reacts with hydrogen bromide via a(n) ___________.Question 8 options:
a) SN1 mechanism
b) SN2 mechanism
c) E1 mechanism
d) E2 mechanism
e) None of these.
Answers
2-Methyl-2-butanol reacts with hydrogen bromide via an SN2 mechanism.
The SN2 (Substitution Nucleophilic Bimolecular) mechanism is a type of reaction in which a substrate molecule is replaced by a nucleophile in a single, concerted step. In this mechanism, the nucleophile attacks the substrate from the backside, pushing the leaving group off and forming a new bond in one step. The reaction rate depends on the concentration of both the substrate and the nucleophile.
In the reaction between 2-methyl-2-butanol and hydrogen bromide, the hydroxyl group in 2-methyl-2-butanol acts as the substrate, while the bromide ion acts as the nucleophile. The bromide ion attacks the substrate from the backside, replacing the hydroxyl group and forming 2-bromo-2-methylbutane. This reaction occurs in a single step and is therefore considered an SN2 mechanism. The reaction is also stereospecific, meaning that it occurs with inversion of configuration at the stereocenter.
Here you can learn more about SN2 mechanism
https://brainly.com/question/7213902#
#SPJ11
In calorimetry, what physical quantity do we measure to qualitatively assess if a reaction is endothermic or exothermic?.
Answers
The physical quantity used to qualitatively assess if a reaction is endothermic or exothermic in calorimetry is heat.
What is calorimetry?Measurements of heat transferred to or from a substance are determined using calorimetry.
Assessment of the reaction:The heat generated by an exothermic reaction in solution is trapped in the calorimeter when we perform the process, raising the temperature of the solution. Running an endothermic reaction causes the solution's temperature to drop as the reaction's heat source is removed from the solution.Characteristics of endothermic reaction:Any chemical process that takes heat from its surroundings is said to be endothermic. The reaction's activation energy comes from the energy that was absorbed. This kind of reaction is characterized by its cold sensation.Characteristics of exothermic reaction:Exothermic simply means releasing heat. As an exothermic process develops, energy, frequently in the form of heat, is released.In an exothermic reaction, it takes less energy to break bonds in the reactants than is released when new bonds form in the products.To learn more about endothermic and exothermic reactions visit:
https://brainly.com/question/10373907
#SPJ4
The physical quantity used to qualitatively assess if a reaction is endothermic or exothermic in calorimetry is heat.
What is calorimetry?Measurements of heat transferred to or from a substance are determined using calorimetry.
Assessment of the reaction:The heat generated by an exothermic reaction in solution is trapped in the calorimeter when we perform the process, raising the temperature of the solution.Running an endothermic reaction causes the solution's temperature to drop as the reaction's heat source is removed from the solution.Characteristics of endothermic reaction:
Any chemical process that takes heat from its surroundings is said to be endothermic.The reaction's activation energy comes from the energy that was absorbed.This kind of reaction is characterized by its cold sensation.Characteristics of exothermic reaction:Exothermic simply means releasing heat.As an exothermic process develops, energy, frequently in the form of heat, is released.In an exothermic reaction, it takes less energy to break bonds in the reactants than is released when new bonds form in the products.To learn more about endothermic and exothermic reactions visit:
brainly.com/question/10373907
#SPJ4
Help please ! Need by tomorrow

Answers
M is equal to (molar mass) (molar volume) (density).
Let's first translate the temperature into Kelvin:
T = 25°C + 273.15 = 298.15 K
The pressure will now be converted to an atm:
760 mm Hg equals 1 atm
Pressure is equal to 740 mm Hg times 760 mm Hg/atm, or 0.9737 atm.
Let's use the ideal gas law equation to determine the molar volume now:
PV = nRT
V is equal to (RT) / P is equal to (0.0821 L atm/mol K) (298.15 K) / (0.9737 atm).
V ≈ 24.93 L/mol
Now, using the provided density, let's get the molar mass:
M = (5.8 g/L) (24.93 L/mol) = (density) (molar volume)
M ≈ 144.394 g/mol
The gas's molar mass is therefore most closely associated with 144.394 g/mol.
The empirical formula for the gas, CH2, reveals the proportional proportion of hydrogen to carbon atoms in the gas. The computed molar mass, however, is considerably more than the molar mass of the empirical formula. This implies that the gas's true molecular composition must have more atoms.
We determine that the ratio is around 3.33 by comparing the molar mass of the empirical formula (14.03 g/mol) to the molar mass of the gas (46.61 g/mol). This suggests that a multiple of the empirical formula makes up the gas' molecular formula.
Learn more about molar mass at :
https://brainly.com/question/12127540
#SPJ1
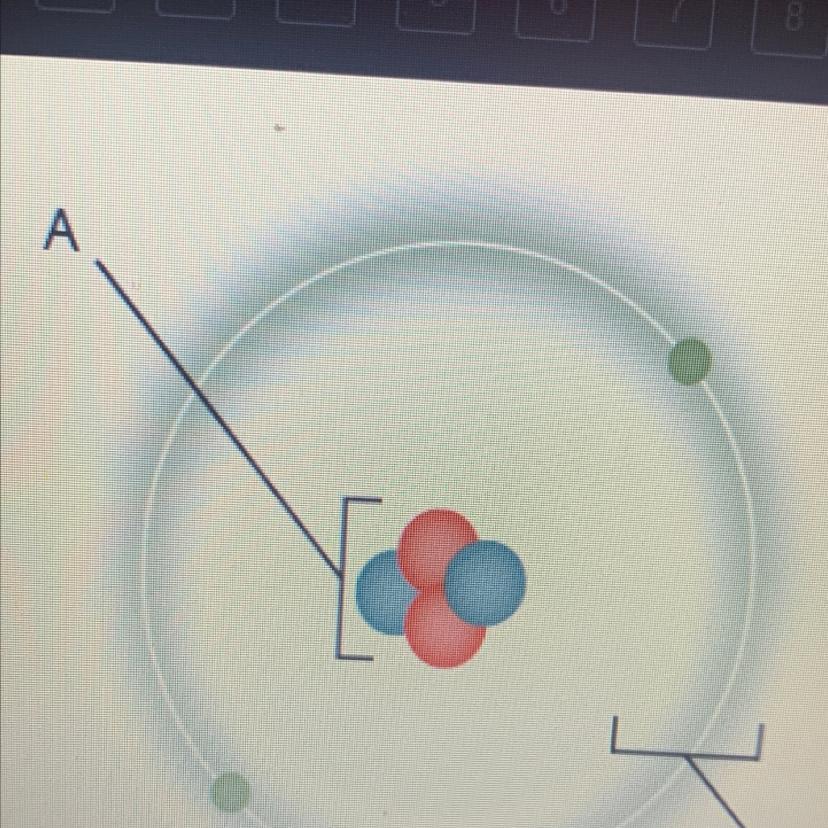
Which is a characteristic of the part of the atom marked “A”?
•it contains most of the mass
•it is negatively charged
•it is mostly empty space
•it is composed of electrons
Answers
Answer:
it contains most of the mass
resonance can only occur when the elements in the molecule keep the same formal charge.a. true b. false
Answers
The given statement "Resonance will only occur when the elements in the molecule keep the same formal charge" is false. Because resonance can occur even if the elements in the molecule do not have the same formal charge in each resonance structure. Option B is correct.
Resonance occurs when there are multiple ways to draw Lewis structures that differ only in the placement of electrons (i.e., the location of double bonds, lone pairs, etc.) but produce the same overall molecule. The formal charges on the individual atoms in the different Lewis structures may vary, but the overall charge of the molecule must remain the same.
For example, consider the nitrate ion (NO₃⁻). The Lewis structure for nitrate ion can be drawn in two different ways, as shown below:
O = N = O⁺ -O⁻
OR
O - N = O
|
O⁻
In the first Lewis structure, the nitrogen atom has a formal charge of +1 and each oxygen atom has a formal charge of -1. In the second Lewis structure, the nitrogen atom has a formal charge of 0 and two of the oxygen atoms have a formal charge of -1.
Hence, B false is the correct option.
To know more about formal charge here
https://brainly.com/question/11723212
#SPJ4
For mn3 , write an equation that shows how the cation acts as an acid.
Answers
Equation showing the Mn³⁺ cation acting as an acid:
Mn³⁺+ H₂O → MnOH²⁺ + H⁺
A cation is a positively charged ion in a metal element whose formation is carried out by releasing one or more electrons from its outermost electrons to achieve stability.
The intermediate reaction is an acid-base reaction. Acid-base reactions generally produce salt and water. However, in this reaction what is formed is an anion and a conjugate acid-base cation because it uses the Bronsted-Lowry acid-base principle. Mn³⁺ acts as an acid so that it donates a proton MnOH²⁺ to H⁺ so that the species formed are MnOH²⁺ and H⁺.
Learn more about cation acts as an acid here https://brainly.com/question/1687261
#SPJ4
In the Diels Alder reaction between 2,3-dimethyl-1,3-butadiene and maleic anhydride to form cyclic anhydride, what was the %recovery using the information below?
Weight of maleic anhydride used: 1 85 mg = 0.1 85g Volume of diene used: 0.215mL Weight of cyclic anhydride crystals recovered: 0.251g % Recovery:
Answers
Recovery = (Weight of product recovered ÷ Theoretical yield) × 100% Recovery = (0.251 g ÷ 0.27594 g) × 100% Recovery = 91.08%Therefore, the % recovery of the reaction between 2,3-dimethyl-1,3-butadiene and maleic anhydride to form cyclic anhydride was 91.08%.
Diels-Alder Reaction Diels-Alder reaction is a chemical process that joins a conjugated diene with a dienophile (a compound containing a double bond) to form a six-membered ring called a cyclohexene ring. It is a chemical reaction that can be used to make new carbon–carbon bonds. The reaction was discovered by two German scientists, Otto Diels and Kurt Alder, in 1928.In the Diels-Alder reaction between 2,3-dimethyl-1,3-butadiene and maleic anhydride to form cyclic anhydride, the % recovery was calculated using the following data:Weight of maleic anhydride used: 185 mg = 0.185 gVolume of diene used: 0.215 mLWeight of cyclic anhydride crystals recovered: 0.251 g% Recovery:We can calculate the percent recovery using the following formula:% Recovery = (Weight of product recovered ÷ Theoretical yield) × 100The theoretical yield of the product can be calculated from the balanced chemical equation as follows:2,3-dimethyl-1,3-butadiene + Maleic Anhydride → Cyclohexene-1,2-dicarboxylic AnhydrideThe molar mass of 2,3-dimethyl-1,3-butadiene is 68 g/mol. The molar mass of Maleic Anhydride is 98 g/mol. The molar mass of Cyclohexene-1,2-dicarboxylic Anhydride is 146 g/mol.Using these values, we can calculate the number of moles of each reactant: moles of diene used = (0.215 mL)(0.788 g/mL)/(68 g/mol) = 0.00248 mol moles of maleic anhydride used = 0.185 g/98 g/mol = 0.00189 mol The theoretical yield of cyclohexene-1,2-dicarboxylic anhydride = 0.00189 mol × 1 mol/1 mol = 0.00189 molThe theoretical yield of cyclohexene-1,2-dicarboxylic anhydride = 0.00189 mol × 146 g/mol = 0.27594 gNow, we can substitute these values into the percent recovery equation:% Recovery = (Weight of product recovered ÷ Theoretical yield) × 100% Recovery = (0.251 g ÷ 0.27594 g) × 100% Recovery = 91.08%Therefore, the % recovery of the reaction between 2,3-dimethyl-1,3-butadiene and maleic anhydride to form cyclic anhydride was 91.08%.
To know more about Diels-Alder Reaction visit :
brainly.com/question/30751490
#SPJ11
Which of these compounds is a proton donor ?
Sodium Sulfate
Nitric acid
Sodium hydroxide
hELP pLEASE
Answers
Answer:
Sodium hydroxide
Explanation:
aaaaaaaaaaaaaaaaaaa
Nitric acid is a proton donor. (Acids are widely known as proton donors and the other cpds are bases).
How many liters of wine can be held in a wine barrel whose capacity is 30.0 gal? You had been given a new penny to test if it is made up of pure copper or not. You measured the mass of the penny which was 2.49 g. You then find that the penny displaces 0.349 cm3 of water. Is the penny made of pure copper? (Density of pure copper = 8.96 g/cm3)
Answers
The first step in this calculation is to know how many liters is equal to 1 gallon, and the value is 3.785 liters, so now we have to make the following calculation:
1 gal = 3.785 Liters
30.0 gal = x Liters
x = 3.785 * 30.0
x = 114 Liters
Three moles of an ideal monatomic gas expands at a constant pressure of 2.50 atm; the volume of the gas changes from 3.20×10
−2
m
3
to 4.50×10
−2
m
3
. Calculate (a) the initial and final temperatures of the gas; (b) the amount of work the gas does in expanding; (c) the amount of heat added to the gas; (d) the change in internal energy of the gas.
Answers
The change in internal energy of the gas is 1505.86 J.
Initial volume = V₁ = 3.20 x 10⁻² m³
Final volume = V₂ = 4.50 x 10⁻² m³
Pressure = P = 2.50 atm
Number of moles = n = 3 mol
Gas constant = R = 8.31 J/mol K
(a) Initial and Final Temperature
The temperature of the gas is given by the ideal gas law:PV = nRT
Initial Temperature :T₁ = PV₁/nR = (2.5 atm x 3.20 x 10⁻² m³) / (3 mol x 8.31 J/mol K) = 321.68 K
Final Temperature:T₂ = PV₂/nR = (2.5 atm x 4.50 x 10⁻² m³) / (3 mol x 8.31 J/mol K) = 458.83 K
(b) Work Done by the gasThe work done by the gas during the expansion can be calculated using the following equation:W = -P∆V
Where ∆V = V₂ - V₁W = -2.50 atm x (4.50 x 10⁻² m³ - 3.20 x 10⁻² m³) = -0.18125 J(c) Heat added to the gas
The first law of thermodynamics relates the change in internal energy (U) of a system to the heat added (Q) to the system and the work done (W) on the system.
∆U = Q - W
where ∆U = change in internal energy = 3/2 nR (∆T) = (3/2) x 3 mol x 8.31 J/mol K (458.83 K - 321.68 K)∆U = 1505.86 JQ = ∆U + WQ = 1505.86 J + (-0.18125 J) = 1505.67875 J(d) Change in Internal Energy
The change in internal energy of the gas can be calculated as:∆U = (3/2) nR (∆T)∆U = (3/2) x 3 mol x 8.31 J/mol K (458.83 K - 321.68 K)∆U = 1505.86 J
Therefore, the initial and final temperatures of the gas are 321.68 K and 458.83 K, respectively.
The work done by the gas during the expansion is -0.18125 J.The amount of heat added to the gas is 1505.67875 J.
The change in internal energy of the gas is 1505.86 J.
Learn more about energy with the given link,
https://brainly.com/question/13881533
#SPJ11
A motorcyclist completes a journey at an average speed of 65 mph
in 3.5 hours. Calculate the distance traveled.
Answers
Hope this helps :)
IS IT BETTER OR WORSE TO WORKOUT/EXERCISE IN DARK OR LIGHT COLORED CLOTHING?
Answers
Answer:
Dark clothing
Explanation:
Dark clothing like black absorbs/locks in heat. Since exercise is to burn and tonify our body from unwanted fat it is important to sweat. This is where heat comes in and helps you sweat
I prefer light coloured cloth to wear, as if we wear dark coloured cloth during exercise it will absorb more heat bcox of the thickness and etc etc..but when it's light coloured cloth it won't have much thickness and it's fabric is just suitable for warm places..
Calculate the mass of solid NaCl that must be added to 1.50 L of a 0.100 M AgNO3 solution to precipitate all the Ag+ ions in the form of AgCl.
Answers
Answer:
0.1169g .
Explanation:
1. Multiply the concentration (0.5 mols/Liters) by the volume of solution you want (0.5 Liters) to find the moles of NaCl you need. 2. Multiply the moles of NaCl by its molar mass (58.44 g/mol) to find the grams of solute needed.
The patient is to receive potassium chloride 40mEq orally. The label states, "Potassium Chloride, 20mEq per 15ml. What volume (ml) will you administer?
Answers
You will need to administer 30ml of potassium chloride to the patient.
To calculate the volume (ml) of potassium chloride to administer, we can use a proportion. The given label states that there are 20mEq of potassium chloride in 15ml.
Let's set up the proportion:
20mEq / 15ml = 40mEq / x ml
To solve for x, cross-multiply:
20mEq * x ml = 15ml * 40mEq
Now divide both sides by 20mEq:
x ml = (15ml * 40mEq) / 20mEq
Simplifying further, we get:
x ml = 30ml
Learn more about potassium chloride from the given link
https://brainly.com/question/31563020
#SPJ11
Examine the fossil. List the parts of the animal that you recognize. What kind of animal do you think this was?

Answers
Answer:
I think it was a huge fish. umm not sure
Explanation:
A sample of paraffin wax undergoes the following change: C25H52(s) → C25H52(l)
(a) During the above process what type of forces, covalent bonds or intermolecular forces, are being broken? Justify your answer
(b) During the change represented above, the density of the sample decreases. Explain this observation on the particulate-level and give clear reference to the meaning of density in your answer.
Answers
Answer:
Explanation:
(a) Answer: Intermolecular forces
The reason for this answer is because the substance (paraffin wax) only changed it's state from solid to liquid and didn't undergo a breakage in it's covalent bond within it's carbon chain which would have produced another substance.
(b) Solid substances are generally more dense than there corresponding liquid substances because the more compact particles are (which occurs in solids), the more dense they become. They are thus more dense than liquids because liquids have there particles loosely packed and well spaced making them less dense than there corresponding solids. Hence, the solid paraffin wax was going to become less dense because it's particles moved from being tightly packed (as solids) to being loosely packed (as liquids). Density refers to mass per volume but can also be described as the level of compactness of a substance. Thus, since liquid is not as compact as solid, it can be said to be less dense than solids.
Which one of the following pairs of substances could be used to construct a single redox
electrode (i.e., they have an element in common, but in different oxidation states)?
A) HCl and Cl- D) Fe3+ and Fe2O3
B) H+ and OH- E) MnO2 and Mn2+
C) H2O and H+
Answers
The pair that could be used to construct a single redox electrode is option E, MnO2 and Mn2+.
Explanation:
Redox electrodes are made up of two different substances, one of which is in a reduced form, and the other is in an oxidized state.
There is an exchange of electrons between the two substances in the redox reaction. In a single redox electrode, the oxidized and reduced forms of the same substance are present.
Redox reactions involve oxidation and reduction of species. Oxidation involves loss of electrons, while reduction involves gain of electrons. Here, MnO2 and Mn2+ have an element in common, i.e., Mn.
The oxidation states of Mn in MnO2 and Mn2+ are +4 and +2, respectively. MnO2 can be reduced to Mn2+ by the addition of electrons. Therefore, MnO2 and Mn2+ can be used to construct a single redox electrode.
To learn more about electrodes, refer below:
https://brainly.com/question/17060277
#SPJ11
The gas carbon dioxide is a pure substance. Which of the following is true about carbon dioxide?
Answers
Answer:
the answer is A :)
Explanation:
Please help answer this. And please no links!

Answers
Answer:
i am not sure but its 2 ican"t qry
Explanation:
Give the formula for the compound formed when magnesium oxide reacts with water. Express your answer as a chemical formula.
Answers
The compound formed when magnesium oxide reacts with water is magnesium hydroxide (Mg(OH)₂).
Magnesium oxide (MgO) is an ionic compound consisting of magnesium cations (Mg²⁺) and oxide anions (O²⁻). When it reacts with water (H₂O), the oxygen atom in water attracts the magnesium cation, leading to the formation of magnesium hydroxide.
The reaction can be represented as follows:
MgO + H₂O → Mg(OH)₂
In the resulting compound, magnesium hydroxide, there are two hydroxide ions (OH-) for every magnesium ion (Mg²⁺). The chemical formula Mg(OH)₂ represents this ratio.
Magnesium hydroxide is an alkaline compound and is commonly used as an antacid to treat heartburn and indigestion. It is also used in various industrial applications.
To know more about magnesium hydroxide, refer here:
https://brainly.com/question/12891796#
#SPJ11
The human body has four types of tissues, and the eye contains all four. Which statement correctly pairs the part of the eye
with the tissue type? (1 point)
Epithelial tissue is found in the sclera, the white part of the eye.
Muscle tissue is found in the iris, the colored part of the eye.
O Connective tissue is found in the comea, the outermost lens of the eye.
Nerve tissue is found in the retina, the rods and cones at the back of the eye.
Answers
Answer:
The correct answer is D) Nerve tissue is found in the retina, the rods and cones at the back of the eye.
Explanation:
The rods and cones are photoreceptors (that is nerve cells within the eyes that can sense light) which is situated in the retina.
These photoreceptors also sense colours and send these signals into the optic nerve for transmission to the brain.
Cheers
A sample of unknown element X contains the following isotopes: 80% of C -64, 15% of X - 65, and 5% of X-66. What is the average atomic mass of element X? Can somebody help me with this question. Will mark brainliest.

Answers
Answer:
64.25
Explanation:
Average atomic mass = percentage × mass of all isotopes/ 100
Relative atomic mass = 80*64+15*65+5*66/100
=5120+975+330/100
=6425/100
64.25
According to the Law of Conservation of Mass, the total
moles of reactants in a balanced chemical equation is...?
O always equal to the total moles of the products.
sometimes more or sometimes less than the total moles of the products.
O always more than the total moles of the products.
O always less than the total moles of the products.
Answers
Answer:
Always equal to the total moles of the products.
Explanation:
The law of conservation of mass states that mass in an isolated system is neither created nor destroyed by chemical reactions or physical transformations. According to the law of conservation of mass, the mass of the products in a chemical reaction equal to the mass of the reactants.
how many moles of barium are in a 25.0 g sample of barium? your answer should have three significant figures.
Answers
1 mole is equal to 6.023 × 10 ²³ molecules. 0.182 moles of barium are in a 25.0 g sample of barium.
What do you mean by mole ?The term mole is defined as the amount of substance of a system which contains as many elementary entities.
One mole of any substance is equal to 6.023 × 10²³ units of that substance such as atoms, molecules, or ions. The number 6.023 × 10²³ is called as Avogadro's number or Avogadro's constant.
The mole concept can be used to convert between mass and number of particles.
I mole of any molecule = 6.023 × 10 ²³
1 mole of barium = 137.328g
To calculate moles, divide mass by molar mass yields number of moles.
25.0g barium × 1mol barium 137.328g barium
= 0.182mol barium
Thus, 0.182 moles of barium are in a 25.0 g sample of barium.
To learn more about the mole, follow the link;
https://brainly.com/question/26416088
#SPJ1
Some students investigate legi’s question. a. Suggest what preliminary work they need to do. b. Draw a table for their results. Include column headings
Answers
Provide a few solutions and explain why they should be effective. Make a succinct conclusion. Provide a succinct summary of what you wrote. A good response.
What might a solution look like?
A good example of a solution is a mixture of salt and sugar. Any solution can be broken down into different parts. Solid, liquid, and gaseous solutions can be classified as solutions based on the physical states of the solvent and solute. Both the solute and the solvent have a solid in solid solutions. For instance, mixtures of polymer and ceramic.
What various categories of water-based solutions are there?
1. Depending on whether the solution contains water or not, different kinds of solutions on water as a solvent can be divided into two categories. the mixture in which freshwater acts as just a solvent and any state of homogenous component totally dissolves in it.
To know more about solutions visit:
https://brainly.com/question/30665317
#SPJ1
Kayla’s teacher gave each lab group three liquids and asked them to test their pH. Kayla tested each one and recorded her results in the chart below.
She then combined the liquids and tested again. She combined liquid C and D and the resulting pH was 7.
Which statement BEST describes the process that took place when liquid C and D were combined?
F) D. The solution would be slightly acidic.
G) C. The acid and base would neutralize to form a salt.
H) A. The acid and base would neutralize to form an element.
I) B. The solution would be slightly basic.
Answers
Answer:
A. The acid and base would neutralize to form an element.
Answer:
egc jnpz rof join it on google meet
the dihydrogenphosphate ion, h2po4? is amphiprotic. in which of the following reactions is this ion serving as a base?
Answers
A substance that can donate a proton (H+) is known as an acid, while one that can accept a proton is known as a base.
The reaction of the dihydrogenphosphate ion with water indicates that it is an amphiprotic substance:H2PO4- + H2O ⇌ H3O+ + HPO42-
The following reaction shows that the dihydrogenphosphate ion is serving as a base:H2PO4- + NH4+ → HPO42- + NH4+H+.
Summary: Hence, the dihydrogenphosphate ion serves as a base in the reaction given as H2PO4- + NH4+ → HPO42- + NH4+H+.
Learn more about acid click here:
https://brainly.com/question/25148363
#SPJ11