Answers
Related Questions
Given the reaction: Mg(s) + 2HCl(aq) → MgCl2(aq) + H2(g)
The reaction occurs more rapidly when a 10-gram sample of Mg is powdered rather than in one piece, because powdered
Mg has
1. less surface area
2. more surface area
3. a lower potential energy
4. a higher potential energy
Answers
Example Scenario:
Leah finished her lunch. All that is left is her plastic sandwich bag. Lea thinks there is no longer anything in the bag, but Paul disagrees. He thinks the bag is filled with air and air is something.
They decide to test their ideas by measuring and comparing the mass and volume of an empty flat and sealed plastic bag with one that has been inflated with air and sealed. Lea thinks that matter does not exist if it cannot be seen. Paul thinks matter can exist even when it's not visible.
Here is the data they collected:

Answers
The data collected by Leah and Paul supports Paul's opinion that matter can exist even when it is not visible.
What is support?Support is a term used to describe a range of services and resources offered to individuals in need. It can refer to providing help with practical tasks, such as helping someone to manage their finances or providing transport to medical appointments. It can also refer to providing emotional or social support. This could involve providing counselling or having regular conversations to help someone to feel understood and supported. Support can also refer to offering advice and guidance, or providing a safe space for someone to talk and express their feelings. In its broadest sense, support is about helping someone to have the best quality of life they can.
This is because the mass of the inflated bag is greater than the mass of the empty bag despite the fact that the volume of the inflated bag is much greater. This shows that the inflated bag contains air, which is matter, even though it cannot be seen.
To learn more about support
https://brainly.com/question/23849325
#SPJ1
1) ¿Cuántos gramos están contenidos en 4 moles de Oro puro Au?
Answers
Answer:
https://sooddan.zendesk.com
Order these chemical species by increasing of an aqueous solution of each. That is, imagine making an solution of each species. Select next to the species that makes the solution with the lowest . Select next to the species that makes the solution with the next higher , and so on. Notice that some of the rankings have been filled in for you already. Also notice that water is on the list. For that particular case, just compare the of pure water to the of the other solutions.
Species Relative PH of 0.1M aqueous solution
HCOOH
CIO2^-
HCOO^-
H2O
H3PO4
H2PO4
H2PO4^-
H3O^-
HClO2
Answers
Answer:
H3O^+ < HClO2 < H3PO4< HCOOH < H2PO4^-<CIO2^-<H2O< HCOO^-
Explanation:
The pH of a solution refers to the negative logarithm of it's hydrogen ion concentration. Generally, the hydrogen ion concentration of an acid is always greater than that of its conjugate base. Hence, the conjugate acid always has a lower pH than the conjugate base.
This can be seen in the species, H3O^+ and H2O, HClO2 and ClO2^- etc.
A 100.0 mL sample of 0.255 M NaOH is mixed with a 100.0 mL sample of 0.200 M HCl in a coffee cup calorimeter. If both solutions were initially at 20.00°C and the temperature of the resulting solution was recorded as 37.00°C, determine the ΔHrxn (in units of kJ/mol HCl) for the neutralization reaction between aqueous NaOH and HCl. Assume 1) that no heat is lost to the calorimeter or the surroundings, and 2) that the density and the heat capacity of the resulting solution are the same as water.
Answers
The ΔHrxn for the neutralization reaction between aqueous NaOH and HCl is -994.6 kJ/mol HCl.
Given that A 100.0 mL sample of 0.255 M NaOH is mixed with a 100.0 mL sample of 0.200 M HCl in a coffee cup calorimeter. Both solutions were initially at 20.00°C and the temperature of the resulting solution was recorded as 37.00°C.
We are to determine the ΔHrxn (in units of kJ/mol HCl) for the neutralization reaction between aqueous NaOH and HCl. The balanced chemical equation for the neutralization reaction between aqueous NaOH and HCl is
NaOH (aq) + HCl (aq) → NaCl (aq) + H2O
(l)First, we need to determine the limiting reactant. It is necessary to identify the limiting reactant in order to calculate the moles of HCl reacted in the reaction and use it to determine ΔHrxn.
Limiting reagent is the reactant that will be completely used up first, stopping the reaction. The reactant that is not completely consumed is the excess reactant.
We can use the concept of Stoichiometry to identify the limiting reactant. To determine the limiting reactant, we can use the following formula:
Molarity (M) = moles of solute (mol) / liters of solution (L) For NaOH, molarity (M) = 0.255 M For HCl, molarity (M) = 0.200 M.
Let's calculate the moles of NaOH and HCl:
Moles of NaOH = Molarity (M) × Volume (L)Moles of NaOH = 0.255 M × 0.100 L = 0.0255 mol
Moles of HCl = Molarity (M) × Volume (L)
Moles of HCl = 0.200 M × 0.100 L = 0.0200 mol
As we can see, the number of moles of NaOH is more than the number of moles of HCl. NaOH is present in excess, while HCl is limiting.
The amount of HCl determines how much NaOH reacts, so we will use the number of moles of HCl to determine ΔHrxn.Next, we can calculate the amount of heat absorbed by the reaction:
qrxn = – qcal where qrxn = heat absorbed by the reactionqcal
= heat released by the calorimeterqcal
= (mass of water + mass of calorimeter) × specific heat of water × ΔTqcal = (200.0 g + 50.0 g) × 4.184 J/g·°C × (37.00°C – 20.00°C)
= 19,892 J or 19.892 kJqrxn
= – 19.892 kJ (because no heat is lost to the calorimeter or the surroundings)
Next, we can use the equation below to calculate ΔHrxn:ΔHrxn = qrxn / n ΔHrxn = -19.892 kJ / (0.0200 mol × (-1)) = 994.6 kJ/mol HCl (Negative sign indicates that the reaction is exothermic).
for such more questions on reaction
https://brainly.com/question/25769000
#SPJ8
how to separate water and cooking oil
Answers
Explanation:
Separatory funnels can be used to separate immiscible liquids. Immiscible liquids are those which won't mix to give a single phase. Oil and water are examples of immiscible liquids. This is because water is more dense than oil, and two layers will thus form. A water layer, and above that, an oil layer.
A separatory funnel has two main components:
A cone shaped glass funnel with hemispherical ends and a neck for a stoppera stopcock at the bottom (a valve which can be opened and closed)A separatory funnel is also usually held above a beaker or flask, with a retort stand and ring clamp.
Consider the separation of water and oil.
Step 1:
The solution is poured into the separatory funnel with the stopcock CLOSED. Due to the immiscible nature and density of the two liquids, they will eventually separate and form two layers.
Step 2:The stopcock is slowly and carefully opened, allowing the bottom layer to flow out. Make sure stopcock is closed JUST before the end of the bottom layer, to ensure there is no contamination of oil with the water in the beaker.
Step 3:Stopcock is opened again, with a new beaker underneath, to allow the little bit of water and a little bit of oil out, to ensure ONLY oil remains in the separatory funnel. Stopcock is closed after this.
Step 4:Stopcock is opened again, with a third clean beaker underneath, to allow ALL of the oil to pass through.
Therefore, you have separated water and oil. See the attached image for a diagram of the setup.
To learn more about the separation of mixtures of immiscible liquids:
https://brainly.com/question/31032868

explain why it is important not to correct any gas from the first few seconds of the experiment
Answers
Answer:
gu kha fuschhehdjdvdbeodbr
Calculate the pOH if the [OH-] concentration is 5.9 x 10_, M? Is the solution ACIDIC, BASIC, or NEUTRAL?
Answers
If the [OH-] concentration is 5.9 x 10^(-M), the pOH of the solution is approximately -4.77 and the solution is basic.
To calculate the pOH of a solution, we can use the formula:
pOH = -log[OH-]
Given that the [OH-] concentration is 5.9 x 10^(-M), we can substitute this value into the formula:
pOH = -log(5.9 x 10^(-M))
Calculating this expression, we find:
pOH = -log(5.9 x 10^(-M))
pOH ≈ -log(5.9) + (-log(10^(-M)))
Since log(10^(-M)) is equal to -M, the equation simplifies to:
pOH ≈ -log(5.9) - M
Now, we need the value of M (the exponent) to calculate the exact pOH value. It appears that the value of M is missing in the given information. However, assuming M is a positive value, we can continue the calculation.
If we consider M = 6, for instance, the equation becomes:
pOH ≈ -log(5.9) - 6
Now, we can evaluate the expression:
pOH ≈ 1.23 - 6
pOH ≈ -4.77
Therefore, if the [OH-] concentration is 5.9 x 10^(-M), the pOH of the solution is approximately -4.77.
To determine whether the solution is acidic, basic, or neutral, we can use the relationship between pH and pOH. The sum of the pH and pOH of a solution at 25°C is always equal to 14.
Since pOH = -4.77, the pH would be:
pH = 14 - pOH
pH ≈ 14 - (-4.77)
pH ≈ 18.77
A solution with a pH above 7 is considered basic. In this case, the calculated pH is greater than 7. Therefore, the solution is basic.
for more questions on pOH
https://brainly.com/question/1420583
#SPJ11
Identify the reducing agent in the following reaction: 2Ca + 2H₂O => Ca(OH)2 + H₂
H₂
H₂O
Ca(OH)2
Ca
Answers
In terms of oxidation number, oxidation can be defined as the chemical change in which there occurs an increase in the oxidation number of an atom. Reduction may be defined as a chemical change in which there occurs a decrease in the oxidation number of an atom. The correct option is D.
An oxidizing agent is a substance which suffers a decrease in the oxidation number of one or more of its elements and a reducing agent is a substance which suffers an increase in the oxidation number of one or more of its elements.
In the reaction, oxidation state of 'Ca' increases from 0 to +2, so calcium is oxidized and acts as a reducing agent.
Thus the correct option is D.
To know more about Reduction, visit;
https://brainly.com/question/1222373
#SPJ1
Which phrases apply to metamorphic rock formation? Check all that apply.
form from existing rocks
form without melting
appear foliated or non-foliated
form from liquid rock
form from deposition
require heat and pressure to form
Answers
Metamorphic rock formation involves the transformation of existing rocks under heat and pressure. They form without melting and can appear foliated or non-foliated.
Explanation:Metamorphic rocks are formed from the transformation of existing rock types in a process called metamorphism, which means 'change in form'. The appropriate phrases that describe metamorphic rock formation are: 'form from existing rocks', 'form without melting', 'appear foliated or non-foliated', and 'require heat and pressure to form'. These rocks are subject to conditions of heat and pressure that cause them to change physically and/or chemically, resulting in a new type of rock. They can either be foliated (layered) or non-foliated. Importantly, metamorphic rock formation does not include a liquid state, meaning they do not 'form from liquid rock' or 'form from deposition'.
Learn more about Metamorphic Rock Formation here:https://brainly.com/question/31426752
#SPJ1
A tank contains 15 kg of dry air and 0.17 kg of water vapor at 30°C and 100 kPa total pressure. Determine
(a) the specific humidity, (b) the relative humidity, and (c) the volume of the tank.
Answers
The volume of the tank is approximately 130.75 m³.
To solve this problem, we need to use the concept of air and water vapor mixture. The given data includes the mass of dry air and water vapor, temperature, and total pressure. We can calculate the specific humidity, relative humidity, and volume of the tank using the following steps:
(a) Specific humidity:
The specific humidity (ω) is defined as the ratio of the mass of water vapor (m_w) to the total mass of the air-water vapor mixture (m_t):
ω = m_w / m_t
Given that the mass of water vapor is 0.17 kg and the total mass of the mixture is 15 kg + 0.17 kg = 15.17 kg, we can calculate the specific humidity:
ω = 0.17 kg / 15.17 kg ≈ 0.0112
So, the specific humidity is approximately 0.0112.
(b) Relative humidity:
Relative humidity (RH) is the ratio of the partial pressure of water vapor (P_w) to the saturation vapor pressure of water (P_ws) at the given temperature, multiplied by 100:
RH = (P_w / P_ws) * 100
To find the relative humidity, we need to determine the saturation vapor pressure at 30°C. Using a vapor pressure table or equation, we can find that the saturation vapor pressure at 30°C is approximately 4.246 kPa.
Given that the total pressure is 100 kPa, the partial pressure of water vapor is 0.17 kg / 15.17 kg * 100 kPa = 1.119 kPa.
Now we can calculate the relative humidity:
RH = (1.119 kPa / 4.246 kPa) * 100 ≈ 26.34%
So, the relative humidity is approximately 26.34%.
(c) Volume of the tank:
To find the volume of the tank, we can use the ideal gas law equation:
PV = nRT
Where P is the total pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature in Kelvin.
First, we need to calculate the number of moles of dry air and water vapor in the tank. The number of moles (n) can be obtained using the equation:
n = m / M
Where m is the mass and M is the molar mass.
The molar mass of dry air is approximately 28.97 g/mol, and the molar mass of water vapor is approximately 18.015 g/mol.
For dry air:
n_air = 15 kg / 0.02897 kg/mol ≈ 517.82 mol
For water vapor:
n_water = 0.17 kg / 0.018015 kg/mol ≈ 9.43 mol
Now we can calculate the volume using the ideal gas law:
V = (n_air + n_water) * R * T / P
Given that R is the gas constant (8.314 J/(mol·K)), T is the temperature in Kelvin (30°C + 273.15 = 303.15 K), and P is the total pressure (100 kPa), we can calculate the volume:
V = (517.82 mol + 9.43 Mol) * 8.314 J/(mol·K) * 303.15 K / (100,000 Pa) ≈ 130.75 m³
for more questions on volume
https://brainly.com/question/29796637
#SPJ8
How many grams are in 0.040 moles zirconium (Zr)?
Answers
Answer:
91.224 gram
Explanation:
Answer:
3.64896 grams Zr
Explanation:
Given: amount of moles of Zr: 0.040
Find: the amount of grams of Zr
1. Find molar mass of Zr
- after looking at the periodic table, you can tell it's 91.224 g/mol
2. Solve
(\(\frac{0.040 mol Zr}{1}\))( \(\frac{91.224 g Zr}{1 mol Zr}\))
- the mol units get cancelled out, and you're left with 0.040 x 91.224 g
3. Multiply that out: 3.64896 grams of zirconium
Which of the following is NOT true of zinc?
-Excess zinc can decrease copper absorption.
-Grains are the most reliable food sources of zinc.
-All of its functions involve it acting as a cofactor for enzymes.
-It binds to most proteins in the body.
Answers
while zinc is an important mineral with numerous functions in the body, it is not true that grains are the most reliable food source of zinc. A balanced diet that includes a variety of foods can provide adequate zinc intake for most people.
How to solve the problem?
The statement that is NOT true of zinc is "Grains are the most reliable food sources of zinc." While grains can be a source of zinc, they are not necessarily the most reliable source.
Zinc is an essential mineral that plays important roles in many biological processes, including immune function, protein synthesis, wound healing, and DNA synthesis. It is involved in various enzymatic reactions, and acts as a cofactor for many enzymes. Zinc is also important for proper growth and development, especially during childhood and adolescence.
Excess zinc intake can lead to decreased copper absorption, as both minerals compete for absorption in the intestines. This can lead to copper deficiency, which can cause anemia neutropenia, and other health problems. Therefore, it is important to maintain a balance between zinc and copper intake.
While grains can be a source of zinc, other foods such as meat, seafood, and dairy products are also good sources. Vegetarians and vegans may need to pay particular attention to their zinc intake, as plant-based sources of zinc may be less bioavailable than -based sources. Zinc supplements can also be used to prevent or treat deficiencies, but should be used with caution as excessive intake can have negative health effects.
In summary, while zinc is an important mineral with numerous functions in the body, it is not true that grains are the most reliable food source of zinc. A balanced diet that includes a variety of foods can provide adequate zinc intake for most people.
To know more about enzymes visit :-
https://brainly.com/question/14577353
#SPJ1
For the reaction
4PH3(g)↽−−⇀6H2(g)+P4(g)
the equilibrium concentrations were found to be [PH3]=0.250 M, [H2]=0.580 M, and [P4]=0.750 M.
What is the equilibrium constant for this reaction?
c=
Answers
The equilibrium constant (Kc) for the given reaction is approximately 16.448. The value of Kc indicates the relative concentrations of reactants and products at equilibrium. In this case, a Kc greater than 1 suggests that the products (H2 and P4) are favored at equilibrium, indicating that the forward reaction is more favorable.
To determine the equilibrium constant (Kc) for the given reaction:
4PH3(g) ↔ 6H2(g) + P4(g)
We can write the equilibrium constant expression based on the stoichiometric coefficients:
Kc = ([H2]^6 * [P4]) / ([PH3]^4)
Substituting the given equilibrium concentrations:
[PH3] = 0.250 M
[H2] = 0.580 M
[P4] = 0.750 M
We can plug in these values into the equilibrium constant expression:
Kc = ([0.580]^6 * [0.750]) / ([0.250]^4)
Kc = (0.0860128 * 0.750) / (0.00390625)
Kc = 16.448
for more question on equilibrium
https://brainly.com/question/18849238
#SPJ8
Identify the activated complex in the following reaction.
a. CuFeSO
b. FeFe
c. FeCuSO4
d. FeSO4
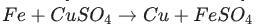
Answers
The activated complex in the following reaction is: FeCuSO4. The activated complex is a transition state that is an intermediate structure in a chemical reaction. Option C)
An activated complex is a structure that exists temporarily during a chemical reaction and corresponds to the top of the energy barrier that must be overcome for the reaction to proceed to completion.
The activated complex in the following reaction is: FeCuSO4. The activated complex is a transition state that is an intermediate structure in a chemical reaction. It is the structure with the greatest energy within the reaction process and is used to determine the rate at which the reaction occurs. An activated complex exists when the energy required to break the old bonds and form new ones has been absorbed. It has a specific configuration and energy content that is precisely defined.
A chemical reaction is the process by which atoms or groups of atoms in molecules interact to form new molecules. A chemical reaction is caused by the motion of electrons, which are negatively charged particles that surround atomic nuclei. The reaction proceeds through the formation of an intermediate species known as the transition state or activated complex. Reaction mechanisms are the sequence of steps involved in a chemical reaction. These steps describe the intermediate species formed as the reactants are converted to products. Hence option C) is correct.
for more questions on reaction
https://brainly.com/question/25769000
#SPJ8
please help!!
62.4 mL of an H2SO3 solution
were titrated with 65.25 mL of a
0.235 M KOH solution to reach the
equivalence point. What is the
molarity of the H2SO3 solution?

Answers
The concentration of H₂SO₃ solution is equal to 0.123 M.
What is a neutralization reaction?A neutralization reaction is described as a reaction in which an acid and base react to form salt and water. When a strong acid will react with a base then the salt which is formed can be neither acidic nor basic.
When H₂SO₃ (a strong acid) reacts with KOH, the resulting salt is K₂SO₃ and water.
H₂SO₃ + 2KOH → K₂SO₃ + 2H₂O
Given, the concentration of KOH = 0.235 M
The volume of the KOH = 65.25 ml = 0.06525 L
The number of moles of KOH, n = M × V = 0.235 × 0.06525 = 0.0153 M
The volume of the H₂SO₃ = 62.4 ml = 0.0624 L
The number of moles of H₂SO₃, n = 0.0153/2 = 0.00767 mol
The concentration of H₂SO₃ =0.00767/0.0624 = 0.123 M
Therefore, the molarity of H₂SO₃ is 0.123 M.
Learn more about neutralization reaction, here:
brainly.com/question/20038776
#SPJ1
Problem: 8.41 g magnesium oxide reacts with 5.40 L of carbon dioxide to form 14.45 g of a compound that is 28.83% magnesium, 14.24% carbon, and 56.93% oxygen. What is the percent yield?
Answers
The yield is 108.2% in percentage. As this result is higher than 100%, it is possible that the experimental process or the measurements had errors.
What occurs when carbon dioxide and magnesium oxide interact?In mild conditions, magnesium oxide works well as a catalyst for the cycloaddition of carbon dioxide to epoxides; when carbon dioxide reacts with (R)-styrene oxide in the presence of magnesium oxide, (R)-phenyl carbonate is produced in 97% of the time with stereochemistry retained.
We must first estimate the theoretical yield of the compound generated in order to calculate the percent yield.
molar mass of MgO = 24.31 g/mol (for Mg) + 15.99 g/mol (for O) = 40.30 g/mol
moles of MgO = 8.41 g / 40.30 g/mol = 0.2087 mol MgO
moles of compound formed = 0.2087 mol MgO = 0.2087 mol compound
mass of compound = (28.83% Mg / 100%) x 14.45 g = 4.16 g Mg
(14.24% C / 100%) x 14.45 g = 2.06 g C
(56.93% O / 100%) x 14.45 g = 8.23 g O
= 14.45 g of the compound formed
percent yield = (actual yield / theoretical yield) x 100%
actual yield = 14.45 g
theoretical yield = 0.2087 mol x (24.31 g/mol + 15.99 g/mol + 3(16.00 g/mol)) = 13.36 g
percent yield = (14.45 g / 13.36 g) x 100% = 108.2%.
To know more about magnesium visit:-
https://brainly.com/question/1533548
#SPJ1
Solids and liquids have higher densities than gases.
Answers
Answer:
yes
Explanation:
in a polymer exchange membrane fuel cell h2 and o2 are combined to give water half cell reactions and overall reaction
Answers
This reaction takes place at the anode and cathode of the fuel cell, with hydrogen (H2) being oxidized at the anode and oxygen (O2) being reduced at the cathode. The flow of electrons (e-) through an external circuit provides the electrical power output of the fuel cell.
The half-cell reactions for hydrogen (H2) and oxygen (O2) in a polymer exchange membrane (PEM) fuel cell are:
Hydrogen half-cell reaction:
2H2(g) -> 4H+(aq) + 4e-
Oxygen half-cell reaction:
O2(g) + 4H+(aq) + 4e- -> 2H2O(l)
The overall reaction in the PEM fuel cell can be obtained by combining the half-cell reactions:
2H2(g) + O2(g) -> 2H2O(l)
Polymer Exchange Membrane (PEM) is a type of membrane used in a variety of applications, particularly in fuel cells and electrolysis. It is made of a thin, ion-conductive film sandwiched between two electrodes. The ion-conductive layer allows for the transfer of ions between the electrodes, while the non-conductive layers serve as a barrier, preventing the mixing of the reactants. PEMs are known for their high proton conductivity, fast ion transport, and stability under extreme conditions.
They are also able to operate at low temperatures, making them well-suited for use in portable and mobile applications. PEMs are typically made of hydrocarbon polymers, such as Nafion, and are typically produced in a thin, flexible sheet. Due to their ability to produce and consume hydrogen, PEMs are an important component in the development of fuel cell technology, which is seen as a promising alternative to traditional energy sources.
To learn more about Polymer exchange membrane (PEM) visit here:
brainly.com/question/29898550
#SPJ4
The Lewis electron-dot structure of oxygen has how many dots?
Answers
Answer:
Oxygen has 6 valence electrons and follows the octet rule.
If you were drawing a lewis structure for O2, there would be a total of 12 valence electrons (6 x 2 = 12).
The completed structure would have a double bond between the two oxygen atoms with 2 lone pairs per oxygen, giving you a total of 8 electrons that are lone pairs (or dots).
HCl(aq) + NaHCO3(aq) → NaCl(aq) + H₂O(l) + CO₂(g)
-
How many moles of CO2 will be produced if I react 300 mL
of 0.4 M HCl solution?
Answers
Answer: 1.333 moles CO2 correct sig figs = 1 mole
Explanation:
1) First solve for moles HCl 2) then do stoichiometry to convert to moles CO2
1) moles = M/L = 0.4 / 0.3 = 1.333 moles HCl
2) 1.333 moles HCl X ( 1moles CO2 / 1 moles HCl) = 1.333 moles CO2
Looking at the web above, each letter represents a species of animal in an ecosystem food web. Which of the following is most likely to cause the greatest decrease in the species B population.
A decrease in species C
A decrease in species E
A decrease in species D
A decrease in species A

Answers
Answer:
a decrease in species A is correct answers
what volume litters of oxygen would be ptoduced in the electrolysis which forms 548 litters of hydrogen both gases measured at stp?
Answers
The ideal gas law may be used to determine the volume of oxygen created in the electrolysis that produces 548 litres of hydrogen at STP (Standard Temperature and Pressure). PV = nRT, where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature, according to the ideal gas equation.
The pressure is 1 atm, the temperature is 273 K, and the number of moles of hydrogen is 548/22.4 = 24.5 in this example. We may compute the volume of oxygen created by rearranging the ideal gas law: V = nRT/P = 24.5*0.082*273/1 = 483.3 litres.
As a result, the volume of oxygen created in the electrolysis at STP that produces 548 litres of hydrogen is 483.3 litres.
Learn more about oxygen at:
https://brainly.com/question/2272415
#SPJ1
How much ice in grams would have to melt to lower the temperature of 352 mL
of water from 15 ∘C
to 0 ∘C
? (Assume that the density of water is 1.0 g/mL
Answers
Answer:
66 grams of ice would have to melt to lower the temperature of 352 mL of water from 15 °C to 0 °C.
Explanation:
To calculate the amount of ice that would have to melt to lower the temperature of 352 mL of water from 15 °C to 0 °C, we need to use the formula:
Q = m_water * c_water * ΔT_water + m_ice * Lf
where,
Q = the amount of heat transferred,
m_water = the mass of water, c_water is the specific heat capacity of water,
ΔT_water = the change in temperature of water, m_ice = the mass of ice,
Lf = the specific latent heat of fusion of ice.
First, let's calculate the amount of heat transferred to the water:
Q = m_water * c_water * ΔT_water
Q = 352 g * 1.0 cal/(g*°C) * (15-0) °C
Q = 5,280 cal
Next, we can use the specific latent heat of fusion of ice, which is 80 cal/g, to calculate the amount of heat required to melt the ice:
Q = m_ice * Lf
Q = m_ice * 80 cal/g
m_ice = Q / Lf
m_ice = 5,280 cal / 80 cal/g
m_ice = 66 g
What happens when an electron jumps from energy level 1 to energy level 2 in an atom?
Answers
The overall process that uptakes energy-poor molecules (CO2 and H2O) from their reservoirs in nature and converts them into energy-rich molecules is
Answers
Answer:
Photosynthesis
Explanation:
Photosynthesis is a process by which photosynthetic organisms use the energy of captured sunlight to convert energy-poor molecules such as carbon (iv), CO₂ and water (H₂O) into energy-rich organic molecules sch as carbohydrates e.g. glucose.
Photosynthesis occurs in a variety of bacteria and algae as well in vascular plants. The overall equation for the reaction of photosynthesis is as follows:
CO₂ + H₂O + light-------> (CH₂O) + O₂
It is a redox reaction in which water donates electrons (as hydrogen) for the reduction of CO₂ to carbohydrate, (CH₂O).
Carbohydrates are energy-rich molecules which serves as energy sources for many living organisms.
Matter is made of atoms that have positive centers of neutrons and protons
surrounded by a cloud of negatively charged electrons.
This statement is...
Answers
If H3O+ were added to a buffer composed of formic acid (HCOOH) and sodium formate (NaHCOO) write the neutralization reaction that would occur in the buffer to resist a change in pH.
Answers
The neutralization reaction that would occur in the buffer to resist a change in pH if H3O+ were added would be:
H3O+ + HCOOH ↔ HCOOH2+
The formic acid (HCOOH) in the buffer would react with the added H3O+ to form the hydronium ion (HCOOH2+), effectively neutralizing the acid and preventing a significant change in pH. This reaction is an example of the buffer capacity of the HCOOH/NaHCOO buffer system. Sodium formate (NaHCOO) would not participate in this neutralization reaction.
If H₃O⁺ were added to a buffer composed of formic acid (HCOOH) and sodium formate (NaHCOO), the neutralization reaction would be:H₃O⁺ + NaHCOO → HCOOH + Na⁺ + H₂O
In this reaction, the formate ion (HCOO⁻) from sodium formate reacts with the added hydronium ion (H₃O⁺) to form formic acid (HCOOH) and water (H₂O), resisting a change in pH.
For more question on neutralization reaction
https://brainly.com/question/23008798
#SPJ11
Some students decide to plant their own garden. They want to eat a more plant-based diet For meat, they will buy locally from a butcher. Any remaining scraps will be composted and used as fertilizer for their garden Which part of this plan represents recycling to reduce their waste?
composting scraps
planting a garden
eating a plant-based diet
buying locally
Answers
Answer:
composting scraps
recycling is the action or process of converting waste into reusable material.
Could I have help with part 2 please (:

Answers
Mass spectrometer M+1 peak is small and m/z unit to the right of the main molecular ion peak
Mass spectrometry is an analytical tool useful for measuring to mass to charge ratio and one or more molecule present in a sample and there measurement can often be used to calculate the exact molecular weight of the sample components as well and there is a technique
Ionization: there are many type of ionization method are used in mass spectrometry method the classic method that most chemist are familiar with are electron impact and fast atom bombardmentDeflection : the ion are then deflected by magnetic field according to their masses and lighter they are the more deflectedDetection : different types of detector are used depending upon factor including dynamic range and special information retention and noise and suitability to the mass analyzerKnow more about peak
https://brainly.com/question/4348492
#SPJ1