Số oxi hoá của nitơ trong NH3, H2SO4,No3 lần lượt là:
Answers
what language is this
Related Questions
The first ionization energies of the elements ______ as you go from left to right across a period of the periodic table, and ______ as you go from the bottom to the top of a group in the table.
A.) increase, decrease
B.) decrease, increase
C.) decrease, decrease
D.) unpredictable, unpredictable
E.) increase, increase
Answers
The correct answer to the question is: A) increase, decrease
The first ionization energies of the elements increase as you go from left to right across a period of the periodic table, and decrease as you go from the bottom to the top of a group in the table.
1. Going from left to right across a period, the atomic number increases, which means there are more protons in the nucleus. This results in a stronger attraction between the positively charged nucleus and the negatively charged electrons. As a result, it becomes harder to remove an electron, requiring more energy, and therefore the first ionization energy increases.
2. Going from the bottom to the top of a group, the atomic size decreases. This is because the number of energy levels or shells decreases, and the electrons are closer to the nucleus. As the distance between the nucleus and the outermost electrons decreases, the attractive force between them increases. Consequently, it becomes easier to remove an electron, requiring less energy, and therefore the first ionization energy decreases.
Therefore, the correct answer to the question is:
A) increase, decrease
To know more about protons, visit:
https://brainly.com/question/12535409
#SPJ11
Following the laser stimulation of the photostimulable phosphor, the excited electrons are ____.
Answers
Photostimulation is a process that includes the absorption of electromagnetic radiation, particularly light, by chromophores or fluorophores to initiate a cellular response. Light-induced physiological responses include vision, plant growth, and photomorphogenesis, among others.
Following the laser stimulation of the photostimulable phosphor, the excited electrons are trapped in the conduction band.What is photostimulation?
Photostimulation is a process that includes the absorption of electromagnetic radiation, particularly light, by chromophores or fluorophores to initiate a cellular response. Light-induced physiological responses include vision, plant growth, and photomorphogenesis, among others.
Laser stimulation
Laser stimulation is the use of laser light to control cellular activity, frequently using ion channels or opsins that have been genetically modified to respond to light. While traditional electrophysiology techniques rely on electrical stimulation of cells, optogenetics employs light as a means of non-invasive and selective manipulation of neural systems or tissues.
Photostimulable
Photostimulable refers to the phosphor's ability to release energy after excitation by light, which allows for energy transfer to the electrons that are trapped in the conduction band. In conclusion, we can say that following the laser stimulation of the photostimulable phosphor, the excited electrons are trapped in the conduction band.
To know more about electromagnetic radiation visit:
https://brainly.com/question/29646884
#SPJ11
you wish to obtain a purified sample of nucleus from lysed cells. the best way to obtain this sample would be
Answers
To obtain a purified sample of the nucleus from lysed cells, the best way is by using a centrifugation technique called differential centrifugation. During differential centrifugation, the cell lysate is spun at different speeds in a series of centrifuges.
The best way to obtain a purified sample of nuclei from lysed cells is through the process of differential centrifugation. Differential centrifugation is a process that involves the separation of different organelles of a cell by subjecting them to centrifugal force at different speeds. In this method, the cell lysate is spun at different speeds in a series of centrifuges.
The first centrifugation is done at low speed, and the resulting pellet is resuspended in a buffer solution and subjected to the second centrifugation at a higher speed. This process is repeated several times, each time at a higher speed. This technique allows for the separation of different cellular components based on their size and density. The final pellet will contain the purified nuclei, which can then be used for further studies or analysis.
You can learn more about differential centrifugation at: brainly.com/question/28287479
#SPJ11
What are 2 indications that a chemical change has occurred?
Answers
Answer: The color has changed or the formation of light and heat. Also, the temprature will change as well.
Explanation:
12.53 Calculate the final concentration of each of the following: a. 2.0 L of a 6.0 M HCl solution is added to water so that the final volume is 6.0 L. b. Water is added to 0.50 L of a 12 M NaOH solution to make 3.0 L of a diluted NaOH solution
Answers
a. The final concentration of the HCl solution is 2.0 M.
The formula to be used is: C1V1 = C2V2, where C1 and V1 are the initial concentration and volume of the HCl solution, respectively, and C2 and V2 are the final concentration and volume of the diluted solution, respectively. Plugging in the values given in the problem, we get: C1V1 = C2V2, or (6.0 M)(2.0 L) = C2(6.0 L). Solving for C2, C2 = (6.0 M)(2.0 L) / (6.0 L) = 2.0 M.
b. The final concentration of the NaOH solution is 2.0 M.
The formula to be used is the same dilution formula as in part (a): C1V1 = C2V2. However, C1 and V1 of the NaOH solution is known, as well as the final volume (V2) of the diluted solution, so, the formula to solve for the final concentration is (C2): C2 = C1V1/V2. Plugging in the values given in the problem, we get: C2 = (12 M)(0.50 L) / (3.0 L) = 2.0 M.
To learn more about concentration, click here:
https://brainly.com/question/10725862
#SPJ11
for each of the reactions at constant pressure, determine whether the system does work on the surroundings, the surroundings does work on the system, or essentially no work is performed.
Answers
Answer:
It is not possible to accurately determine whether the system or the surroundings performs work in a chemical reaction without more information about the specific reaction and the conditions under which it occurs.
In general, the work done in a chemical reaction can be affected by several factors, including the pressure, volume, and temperature of the system, as well as the nature of the reactants and products. Some reactions may result in the system doing work on the surroundings, such as when gases expand and do work on their surroundings by pushing against a piston or other external object. Other reactions may result in the surroundings doing work on the system, such as when gases are compressed and their surroundings do work on them. Still other reactions may result in essentially no work being performed, such as when the reactants and products are in equilibrium or when the volume of the system remains constant.
Without more information about the specific reaction in question, it is not possible to accurately determine whether the system or the surroundings performs work.
Plz solve mcqs#04 with full detailed.note

Answers
Answer:
The correct option is;
(B) 1 s², 2s², 2p⁶, 3s², 3p³
Explanation:
The electron configuration is the outline of the electron arrangement about a nucleus
In the systemic pattern of electron arrangement within an atom, there are, s, p, d, f orbitals
The maximum number of electrons in an s, p and d orbital = 2, 6, and 10 respectively
Based on Aufbau's principle the electrons are arranged based on the order of their energy level
The charge is presented by the number of electrons in the outermost shell, an element able to form an ion of charge of -3 will gain 3 electrons to complete its outermost shell
Among the options given, option B is the only option that has the capacity to take the electrons to complete the number of electrons in the p orbital outermost shell to 6 from 3, that is 3p³ + 3e⁻→ 3p⁶.
___+___=__KOH+H2 It Is chemistry question
Answers
Answer:
It's from the quation:
\( \boxed{ \mathsf{ \underline{2K} +\underline{2 H_2O} \rightarrow \:\underline{2}KOH + H_2 }}\)
(How did I know?)
I learnt it while studying the elements of group 1.
- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - -
Group 1 elements:K, here, is Potassium. It's position in the periodic table is Group 1, Period 4.
Group 1 elements are also called Alkali metals. Group 1 elements have 1 electron in their valence shell(outermost shell), the electron that takes part in chemical reactions.Valance electrons of elements of this group contribute to the valency of the group's elements I.e.,
valency of Alkali metals is 1!
- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - -
Reaction with water:Alkali metals react vigorously with cold water to form their hydroxides alongwith the evolution of Hydrogen gas.What are hydroxides?
Hydroxides of an element comprises one atom each of oxygen and Hydrogen bonded together, acting as an anion, the hydorxide anion — OH-.
Example:
KOH (hydroxide of Potassium) NaOH (Hydroxide of Sodium)- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - -
Back to the question:In the question, The reactants are missing but the products are known, that are, Potassium Hydroxide and water.
Keeping the above mentioned property in mind, the reactants come clear as Potassium(K) and Cold water(H2O).
that makes the equation:
\( \mathsf{K + H_2O \rightarrow \: KOH + H_2 }\)
- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - -
Balancing the equation:For an equation to be balanced, the number of atoms of each elements on both side of the equation must be equal.
Number of atoms of each element on the LHS:
K = 1 H = 2O = 1(subscript in front of an atom represents its number of atoms in the compund)
Number of atoms of each element on the LHS:
K = 1 H = 1 + 2 = 3O = 1The number of Hydrogen atoms on boths sides is NOT balanced!
Hit and trial method:
If we add a "2" in front of K, H2O and KOH
\( \mathsf{ \underline{2K} +\underline{2 H_2O} \rightarrow \:\underline{2}KOH + H_2 }\)
Number of atoms on both the sides become equal.
(TIP: This comes thru practice:/. but main point is even out the oxygen and Hydrogen atoms first. like if it's 3 multiply it by 2 and you get an even number, I.e., 6)
Number of atoms of each element on the LHS:
K = 2H = 4O = 2Number of atoms of each element on the LHS:
K = 2H = 2 + 2 = 4 O = 2- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - -
Answer:Hence, the balanced form of the equation is:
\( \boxed{ \mathsf{ \underline{2K} +\underline{2 H_2O} \rightarrow \:\underline{2}KOH + H_2 }}\)
What is the organism with modified genome called
Answers
Answer:(GMO)
Explanation: genetically modified organism (GMO)
an atom with six protons and eight neutrons is a(n)___
a. isotope of nitrogen
b. standard carbon atom
c. isotope of carbon
d. isotope of oxygen
Answers
Answer:
standard carbon atom
Explanation:
Answer:
Explanation:
This is an isotope of carbon, more specifically, carbon-14. Carbon 14 has 6 protons, making the element carbon, and eight neutrons since 14-6 = 8.
a product that donates electrons to the electron transport chain
Answers
The product that donates electrons to the electron transport chain is NADH.
The electron transport chain (ETC) is a series of chemical reactions that occur within the inner mitochondrial membrane. It's a process that generates adenosine triphosphate (ATP), which is the energy currency of the cell. NADH is produced during the process of glycolysis and the citric acid cycle. It is a molecule that carries electrons and hydrogen ions (H+) to the electron transport chain in the mitochondria.
During the process of cellular respiration, NADH donates electrons to the electron transport chain, which facilitates the pumping of H+ ions across the inner mitochondrial membrane .This results in a concentration gradient of H+ ions, which powers the ATP synthase enzyme. This enzyme converts ADP (adenosine diphosphate) to ATP by adding a phosphate group. The donation of electrons by NADH to the electron transport chain is a crucial step in the process of cellular respiration.
To learn more about NADH:
https://brainly.com/question/32157850
#SPJ11
Chemisrty seeks to explain the submicroscopic events that lead to macroscopic observations. explain this statement
Answers
Submicroscopic events are the structure and behavior of the matter or particle that can use for the macroscopic observations of the matter.
What are submicroscopic elements?Sub microscopic elements are very minute and small particles that can be only seen by a microscope. These are small particles that together make the big matter.
Macroscopic particles are big particles that are constituted by small particles.
Thus, the structure and behavior of the matter or particle that can be used for macroscopic observations of the matter are submicroscopic occurrences.
To learn more about submicroscopic elements, refer to the link:
https://brainly.com/question/28243921
#SPJ4
An E. coli merodiploid has the following genotype:
lacl+ laco* lacZ+ lacy lacA+ / F' lac laco lacZ lacy+ lacA+
What is this strain's phenotype in the absence (-) or presence (+) of IPTG? A.- IPTG: LacZ+ LacY- LacA+
+ IPTG: LacZ+ LacY- LacA+ B. - IPTG: LacZ- LacY+ LacA+
+ IPTG: LacZ- LacY+ LacA+ C.- IPTG: LacZ- LacY+ LacA+
+ IPTG: LacZ- LacY+ LacA+ D.- IPTG: LacZ+ LacY- LacA+
+ IPTG: LacZ+ LacY+ LacA+ E. IPTG: LacZ- LacY+ LacA+ -
+ IPTG: LacZ+ LacY+ LacA+
Answers
Since there are mutations in the lacZ and lacY genes (lacz- and lacy-), only the LacA protein, encoded by the laca+ allele, is functional. Therefore, the phenotype is Lacz- LacY+ LacA+.
Based on the given genotype, the phenotype of the E. coli merodiploid strain in the absence (-) or presence (+) of IPTG can be determined as follows:
IPTG: Lacz- LacY- LacA+
IPTG: Lacz- LacY+ LacA+
In the absence of IPTG, the lac operon is not induced, and the lac repressor protein encoded by the lacl° allele is non-functional. Therefore, it cannot bind to the operator region, allowing the transcription of lacZ, lacY, and lacA genes. However, since there are mutations in the lacZ and lacY genes (lacz- and lacy-), the LacZ and LacY proteins are not produced. The LacA protein, encoded by the laca+ allele, is functional, resulting in the phenotype Lacz- LacY- LacA+.
IPTG: Lacz- LacY+ LacA+
In the presence of IPTG, IPTG acts as an inducer of the lac operon. It binds to the repressor protein encoded by the lacl° allele, causing a conformational change that prevents it from binding to the operator. This allows transcription of the lacZ, lacY, and lacA genes. However, since there are mutations in the lacZ and lacY genes (lacz- and lacy-), only the LacA protein, encoded by the laca+ allele, is functional. Therefore, the phenotype is Lacz- LacY+ LacA+.
So, the correct answer is A. - IPTG: Lacz- LacY- LacA+
IPTG: Lacz- LacY+ LacA+.
Learn more about Transcription here:
brainly.com/question/29739443
#SPJ11
Nitrogen dioxide is one of the many oxides of nitrogen (often form another form of NOx, dinitrogen tetroxide A chemical engineer studying this reaction fils a 500. ML flask at 7. 9 °C with 4. 9 atm of nitrogen dioxide gas. He thèn raises the temperature considerably and when the mixture has come to equilibrium determines that it contains 2. 7 atm of nitrogen dioxide gas The engineer then adds another 1. 2 atm of nitrogen dioxide, and allows the mixture to come to equilibrium again. Calculate the pressure of dinitrogen tetroxide after equilibrium is reached the second time. Round your answer to 2 significant digits collectively called·N ' that are of interest to atmospheric chemistry. It can eact with ter to 囲 atm □-10 I Don't Know Submit
Answers
When the engineer adds an additional 1.2 atm of nitrogen dioxide, the total pressure of the mixture is 4.9 atm + 1.2 atm = 6.1 atm. At equilibrium, the pressure of dinitrogen tetroxide is approximately 2.3 atm.
This is calculated by subtracting the equilibrium pressure of nitrogen dioxide (2.7 atm) from the total pressure of the system (6.1 atm). Therefore, after equilibrium is reached the second time, the pressure of dinitrogen tetroxide is 2.3 atm.
When nitrogen dioxide gas is added to a system, the equilibrium pressure of dinitrogen tetroxide is determined by Le Chatelier's principle. This principle states that when a system is disturbed from equilibrium, it will shift to re-establish equilibrium.
Learn more about Nitrogen dioxide:
https://brainly.com/question/9183984
#SPJ4
CO2(g) + H2O(I) - -> C6H12O6(s) + O2(g)
How many of the chemicals in the equation above are aqueous? #only
Answers
Answer:
For instance equation C6H5C2H5 + O2 = C6H5OH + CO2 + H2O will not be balanced, but PhC2H5 + O2 = PhOH + CO2 + H2O will; Compound states [like (s) (aq) or (g)] are not required. If you do not know what products are enter reagents only and click 'Balance'. In many cases a complete equation will be suggested.
Explanation:
The density of a gas is the mass per unit volume of the gas in the units of, for example, grams per litre. By finding the mass of one litre (assume 1.00L) of gas you can calculate the density of the gas. knowledge of the densities of the gas compared to the density of air (1.2 g/l), can save your life.
A) what is the density of carbon monoxide gas at 20C and 98 kPa in your home.
Answers
The density of carbon monoxide gas at 20°C and 98 kPa is 1.145 g/L.
The ideal gas law is PV = nRT
where P is the pressure, V is the volume, n is the number of moles of gas, R is the gas constant, and T is the temperature in kelvin.
To find the density of carbon monoxide gas at 20°C and 98 kPa, we can use the ideal gas law to find the number of moles of gas in 1 L of gas at these conditions and then divide the mass of 1 mole of gas by the number of moles to get the density.
First, we need to convert the temperature to kelvin:
20°C + 273.15 = 293.15 K
Rearranging the ideal gas law, we get:
n = PV/RT
We can assume that the volume is 1 L, so:
n = (98 kPa)(1 L) / [(0.0821 L·atm/mol·K)(293.15 K)] = 0.0413 mol
The molar mass of carbon monoxide is 28.01 g/mol, so the mass of 0.0413 mol is:
0.0413 mol x 28.01 g/mol = 1.152 g
Therefore, the density of carbon monoxide gas at 20°C and 98 kPa is:
1.152 g / 1 L = 1.145 g/L
What is density?
Density is a physical property of matter that relates to the amount of mass per unit of volume of a substance. It is typically expressed in units such as grams per cubic centimeter (g/cm³) or kilograms per cubic meter (kg/m³).
To know more about density, visit:
https://brainly.com/question/29775886
#SPJ1
Please dont answer if you dont know.
It's the parts of a wave
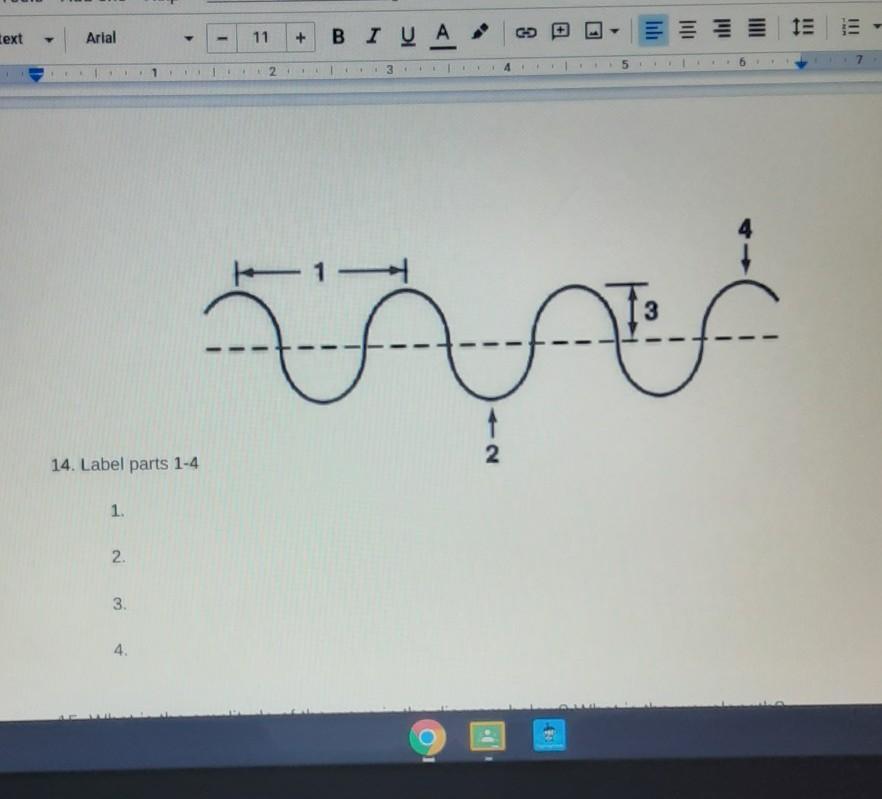
Answers
1) wavelength
2) trough
3) amplitude
4) crest
Explanation:
Hope this helps!
Calculate the ΔG°rxn using the following information.
2 HNO3(aq) + NO(g) → 3 NO2(g) + H2O(l) ΔG°rxn=?
ΔH°f (kJ/mol) -207.0 91.3 33.2 -285.8
S°(J/mol∙K) 146.0 210.8 240.1 70.0
A) -151 kJ
B) -85.5 kJ
C) +50.8 kJ
D) +222 kJ
E) -186 kJ
Answers
To calculate the standard Gibbs free energy change (ΔG°rxn) for the given reaction, we can use the equation:ΔG°rxn = ΔH°rxn - TΔS°rxn, Given: ΔH°f (kJ/mol) values:HNO3(aq): -207.0 kJ/mol, NO(g): 91.3 kJ/mol, NO2(g): 33.2 kJ/mol and H2O(l): -285.8 kJ/mol.
S° (J/mol∙K) values:
HNO3(aq): 146.0 J/mol∙K
NO(g): 210.8 J/mol∙K
NO2(g): 240.1 J/mol∙K
H2O(l): 70.0 J/mol∙K
Let's calculate the ΔH°rxn:
ΔH°rxn = [3 × ΔH°f(NO2(g))] + [ΔH°f(H2O(l))] - [2 × ΔH°f(HNO3(aq))] - [ΔH°f(NO(g))]
ΔH°rxn = [3 × 33.2 kJ/mol] + [-285.8 kJ/mol] - [2 × (-207.0 kJ/mol)] - [91.3 kJ/mol]
ΔH°rxn = 99.6 kJ/mol - 285.8 kJ/mol + 414.0 kJ/mol - 91.3 kJ/mol
ΔH°rxn = 136.5 kJ/mol
Calculate the ΔS°rxn:
ΔS°rxn = [3 × S°(NO2(g))] + [S°(H2O(l))] - [2 × S°(HNO3(aq))] - [S°(NO(g))]
ΔS°rxn = [3 × 240.1 J/mol∙K] + [70.0 J/mol∙K] - [2 × 146.0 J/mol∙K] - [210.8 J/mol∙K]
ΔS°rxn = 720.3 J/mol∙K + 70.0 J/mol∙K - 292.0 J/mol∙K - 210.8 J/mol∙K
ΔS°rxn = 287.5 J/mol∙K
Now, we can calculate ΔG°rxn using the equation:
ΔG°rxn = ΔH°rxn - TΔS°rxn
If we assume a standard temperature of 298 K, we can substitute the values: ΔG°rxn = 136.5 kJ/mol - (298 K * 0.2875 kJ/mol∙K)
ΔG°rxn = 136.5 kJ/mol - 85.57 kJ/mol
ΔG°rxn ≈ 50.93 kJ/mol
The calculated ΔG°rxn is positive (+50.93 kJ/mol). Therefore, based on the given options, the closest answer is: +50.8 kJ
Learn more about standard Gibbs free energy here ;
https://brainly.com/question/30654218
#SPJ11
Ayuda por favor no entiendo nada voy a reprobar 2- Completa el siguiente cuadro indicando la cantidad de partículas subatómicas fundamentales para cada uno de los siguientes átomos: Atomos | N° atómico | N° de protones | N° de electrones | N° de neutrones 7 Li 20Ne 35Cl 31P 80Br 14C
Answers
Answer:
Explanation:
Litio -7 (⁷₃Li)
número de protones = 3
número de electrones = 3
número de neutrones = 4
Neon- 20 (²⁰₁₀Ne)
número de protones = 10
número de electrones = 10
número de neutrones = 10
Cloro - 35 (³⁵₁₇Cl)
número de protones = 17
número de electrones = 17
número de neutrones = 18
Fósforo - 31 (³¹₁₅P)
número de protones = 15
número de electrones = 15
número de neutrones = 16
Bromo - 80 (⁸⁰₃₅Br)
número de protones = 35
número de electrones = 35
número de neutrones = 45
Carbono - 14 (¹⁴₆C)
número de protones = 6
número de electrones = 6
número de neutrones = 8
Why did Cyrus Field want to build a transatlantic telegraph?
Answers
In 1854, Cyrus West Field conceived the idea of the telegraph cable and secured a charter to lay a well-insulated line across the floor of the Atlantic Ocean. Obtaining the aid of British and American naval ships, he made four unsuccessful attempts, beginning in 1857.
a) Aluminum metal reacts with iron (l) oxide powder to produce aluminum oxide solid and iron metal.
Answers
Answer:
\(\boxed{\rm Al_{\,(s)}+Fe_2O_{\,(s)} \rightarrow Al_2O_{3\,(s)} + Fe_{\,(s)}}\)
Explanation:
Aluminium metal + iron(I) oxide powder → aluminium oxide solid + iron metal
This is an example of a displacement reaction, with chemical equation:
\(\boxed{\rm Al_{\,(s)}+Fe_2O_{\,(s)} \rightarrow Al_2O_{3\,(s)} + Fe_{\,(s)}}\)
To learn more about displacement reactions:
https://brainly.com/question/13219117
Select the compound that is soluble in water?

Answers
Answer:
NaOH
Explanation:
NaOH is among the water soluble bases
Help please fast as possible I’ll mark you brainlist

Answers
Answer:
As mass increases the gravitational force increases
Explanation:
Things with mass gain a gravitational force, The bigger they are the more force, for example: the sun is the biggest so it pull us
Write the cell notation for an electrochemical cell consisting of an anode where Pb (s) is oxidized to Pb2 (aq) and a cathode where Fe3 (aq) is reduced to Fe2 (aq) at a platinum electrode . Assume all aqueous solutions have a concentration of 1 mol/L and gases have a pressure of 1 bar.
Answers
The cell notation for the electrochemical cell with a Pb(s) anode oxidized to Pb²⁺(aq) and a platinum electrode cathode where Fe³⁺(aq) is reduced to Fe²⁺(aq) is:
Pb(s)|Pb²⁺(aq, 1M)||Fe³⁺(aq, 1M), Fe²⁺(aq, 1M)|Pt(s)
The cell notation for the given electrochemical cell can be represented as follows:
Pb(s)|Pb²⁺(aq, 1M)||Fe³⁺(aq, 1M), Fe²⁺(aq, 1M)|Pt(s)
In this notation, the single vertical line "|" represents a phase boundary, while the double vertical lines "||" represent the salt bridge that connects the two half-cells. The anode (where oxidation occurs) is written on the left, and the cathode (where reduction occurs) is written on the right.
For more such questions on cell notation, click on:
https://brainly.com/question/17218591
#SPJ11
what kind of oxide is formed when a piece of sodium is dropped in the water
Answers
Answer:
Sodium oxide is the product
Explanation:
4Na+O2->2Na2O
1)26.4 % Carbon
3.3 % Hydrogen
70.3 % Oxygen
Molar Mass: 91.0 g/mol
Empirical Formula:
Molecular Formula:
Answers
Answer:
1. Empirical formula => CH2O2
2. Molecular formula => C2H4O4
Explanation:
From the question given above, the following data were obtained:
Carbon (C) = 26.4 %
Hydrogen (H) = 3.3 %
Oxygen (O) = 70.3 %
Molar mass of compound = 91.0 g/mol
Empirical formula =..?
Molecular formula =..?
1. Determination of the empirical formula of the compound.
C = 26.4 %
H = 3.3 %
O = 70.3 %
Divide by their molar mass
C = 26.4 /12 = 2.2
H = 3.3 /1 = 3.3
O = 70.3 /16 = 4.39
Divide by the smallest
C = 2.2 /2.2 = 1
H = 3.3 /2.2 = 2
O = 4.39 /2.2 = 2
Empirical formula => CH2O2
2. Determination of the molecular formula of the compound.
Molar mass of compound = 91.0 g/mol
Empirical formula => CH2O2
Molecular formula => [CH2O2]n
We shall determine the value of n as follow:
[CH2O2]n = 91
[12 + (2×1) + (2×16)]n = 91
[12 + 2 + 32]n = 91
46n = 91
Divide both side by 46
n = 91/46
n = 2
Molecular formula => [CH2O2]n
Molecular formula => C2H4O4
In the given case where 26.4 % Carbon , 3.3 % Hydrogen , 70.3 % Oxygen , and Molar Mass: 91.0 g/mol the:
Empirical Formula: \(C_2H_3O_4\) Molecular Formula: (\(C_2H_3O_4\))\(_1\)Given:
Carbon (C) = 26.4 %
Hydrogen (H) = 3.3 %
Oxygen (O) = 70.3 %
Molar mass of compound = 91.0 g/mol
Determination of the empirical formula of the compound:-
Divide by their molar mass for obtaining moles: C = 26.4 /12 = 2.2 H = 3.3 /1 = 3.3 O = 70.3 /16 = 4.39 for ratio Divide by the smallest: C = 2.2 /2.2 = 1 H = 3.3 /2.2 = 1.5 O = 4.39 /2.2 = 2
The ratio is CHO = 1 : 1.5 : 2
multiply with 2 to find correct and complete number ratio
C = 1 × 2 = 2
H = 1.5 × 2 = 3
O = 2 × 2 = 4
Thus, the Empirical formula => \(C_2H_3O_4\)
Mass × n = molar mass
12 × 2 + 1 × 3 + 16 × 4 = 91
24 + 3 + 64 = 91
91 = 91
Thus moles are 1 which means
molecular formula = (\(C_2H_3O_4\))\(_1\)
Thus, here in the given data:
Empirical Formula: \(C_2H_3O_4\)Molecular Formula: (\(C_2H_3O_4\))\(_1\)
Learn more:
https://brainly.com/question/15960587
Determine the mass in grams of each of the following:
a. 3.00 mol Al
b. 2.56 × 10^24 atoms Li
c. 1.38 mol N
d. 4.86 × 10^24 atoms Au
e. 6.50 mol Cu
f. 2.57 × 10^8 mol S
g. 1.05 × 10^18 atoms Hg
Answers
Answer:
a. 3.00 mol Al = 3.00 x 27.0 g/mol = 81.0 g
b. 2.56 × 10^24 atoms Li = 2.56 × 10^24 x 6.939 g/mol = 17.75 g
c. 1.38 mol N = 1.38 x 28.0 g/mol = 38.64 g
d. 4.86 × 10^24 atoms Au = 4.86 × 10^24 x 197.0 g/mol = 961.3 g
e. 6.50 mol Cu = 6.50 x 63.5 g/mol = 410.75 g
f. 2.57 × 10^8 mol S = 2.57 × 10^8 x 32.1 g/mol = 82,567,600 g
g. 1.05 × 10^18 atoms Hg = 1.05 × 10^18 x 200.6 g/mol = 210,000 g
please help me. :)))

Answers
46.12 grams of water are produced when 35 grams of \(C_6H_1_0\) react with 45 grams of \(O_2\).
Stoichiometric problemThe balanced equation for the combustion of C6H10 (cyclohexene) with O2 is:
\(C_6H_{10} + O_2 - > CO_2 + H_2O\)
From the balanced equation, we can see that 1 mole of \(C_6H_1_0\) reacts with 6 moles of O2 to produce 6 moles of water.
First, let's calculate the number of moles of C6H10 and O2:
Molar mass of C6H10 = 6(12.01 g/mol) + 10(1.01 g/mol) = 82.16 g/mol
Number of moles of C6H10 = mass / molar mass = 35 g / 82.16 g/mol ≈ 0.426 mol
Molar mass of O2 = 2(16.00 g/mol) = 32.00 g/mol
Number of moles of O2 = mass / molar mass = 45 g / 32.00 g/mol ≈ 1.406 mol
From the stoichiometry of the balanced equation, we can determine that 0.426 moles of C6H10 will produce 0.426 * 6 = 2.556 moles of water.
Now, let's calculate the mass of water produced:
Molar mass of H2O = 2(1.01 g/mol) + 16.00 g/mol = 18.02 g/mol
Mass of water = number of moles of water * molar mass of water
= 2.556 mol * 18.02 g/mol
≈ 46.12 g
Therefore, approximately 46.12 grams of water are produced when 35 grams of C6H10 reacts with 45 grams of O2.
More on stoichiometric problems can be found here: https://brainly.com/question/32305503
#SPJ1
on the periodic table of the elements, mercury (hg) has an atomic number of 80 and a mass number of 200.59. it has seven stable isotopes. the most abundant of these probably have
Answers
Mercury (Hg) has an atomic number of 80 and a mass number of 200.59. It has seven stable isotopes. The most abundant of these probably have more than 80 neutrons each.
Option A is correct.
What does the term "atomic number" mean?The number of a chemical element in the periodic system, in which the elements are arranged by the number of protons in the nucleus, which increases with increasing number. As a result, the atomic number is also the number of protons in the neutral atom, which is always the same as the number of electrons. The number of protons in an atom's nucleus is known as its atomic number. An element's identity is determined by its number of protons—for example, a carbon atom with six protons is an element, regardless of how many neutrons are present.
An atom's atomic number is the same as the number of protons in its nucleus or electrons in an electrically neutral atom. The number of protons in an atom. A sodium atom, for instance, has 11 electrons and 11 protons.
Incomplete question :
On the periodic table of the elements, mercury (Hg) has an atomic number of 80 and a mass number of 200.59. It has seven stable isotopes. The most abundant of these probably have ________.
A. more than 80 neutrons each.
B. more than 50 neutrons each
C. less than 80 neutrons each
Learn more about atomic number :
brainly.com/question/11353462
#SPJ1
Can an oxygen tank ever be half empty?
Answers
Answer is yes because to say that the tank is half empty would mean that all the oxygen molecules only occupy half or less of the tank.