. SE7.12D Which of the following statements accurately represents the differences in
plant and animal cells?
Only plant cells have a cell wall, chloroplasts, and a large vacuole.
Only animal cells have a cell wall, chloroplasts, and a large vacuole.
Only plant cells have a cell membrane, mitochondria, and cytoplasm.
Only animal cells have a cell membrane, mitochondria, and
cytoplasm.
CLEAR ALL
Answers
Only plant cells have a cell wall, chloroplasts, and a large vacuole.
I hope this helped :)
Features which are found in plant cells only are the chloroplast, large vacuole and cell wall.
Plants and animal cells consists of the following parts ; Mitochondria, cell membrane, vacuole, cytoplasm and so on. The vacuole present in animal cells are much smaller than those found in plant Cells. Hence, vacuole in animal cells are called small vacuole Plant cells also consists of cell wall, chloroplast and a large vacuole. All these are missing in animal cells.Therefore, Only plant cells have "a cell wall, chloroplasts and a large vacuole."
Learn more : https://brainly.com/question/18012076?referrer=searchResults
Related Questions
Which evidence cannot be found in spoiled foods?
a. production of gas
b. change of color
c. change of state
d. precipitate found
Answers
Answer:
a. Change of state
Explanation:
Because you will see that the state has changed
Which condition would cause surface runoff to increase in a particular location?
1. Covering a dirt road with pavement.
2. Planting grasses and shrubs on a hillside.
3. Having a decrease in the annual rainfall.
Answers
Answer:
number 1
Explanation:
the water in a beaker has a volume of 50 millimeters, is this an extensive property?
Answers
No, the volume of water in a beaker is not an extensive property.
Extensive properties are those that depend on the amount or size of the substance being measured. In other words, they are properties that change with the quantity of the substance. Examples of extensive properties include mass, volume, and total energy.
In the given scenario, the volume of water in the beaker is 50 milliliters. This volume remains the same regardless of the quantity of water present. Whether it's 50 milliliters or 500 milliliters, the volume measurement does not change. Therefore, the volume of water in the beaker is an example of an intensive property.
Intensive properties are independent of the amount or size of the substance. They are characteristics that remain constant regardless of the quantity of the substance. Examples of intensive properties include temperature, density, and color.
It's important to note that the distinction between extensive and intensive properties depends on the specific property being considered. While volume is typically an extensive property for a bulk substance, in the case of a fixed volume of water in a beaker, it becomes an intensive property.
In summary, the volume of water in a beaker is not an extensive property but rather an intensive property because it does not change with the quantity of the substance.
For more such questions on extensive property visit:
https://brainly.com/question/13055036
#SPJ8
How can you separate sand and sugar?
Answers
Answer:
1)Filtration to remove sand
2)Evaporation to obtain sugar
Explanation:
Add water to the mixture and mix,sugar will dissolve.
Filter out the sand.
Evaporate the remaining solution to obtain the sugar.
How many calories are there in 32 Calories?
a.64,000
b.16,000
C.32,000
D..032
Answers
Answer:
32
Explanation:
There cannot be more in a number than the number. Therefore, the answer has to be D, or 32.
Hope this helps! :)
PLESE HELP! Which of the following sugar solutions is the most concentrated?
Select one:
a. 25 g of sugar in 60 mL of water
b. 2 g of sugar in 100 mL of water
c. 15 g of sugar in 25 mL of water
d. 12 g of sugar in 30 mL of water
Answers
c.15gof in25mlof wather
The sugar solution that is the most concentrated is 15 g of sugar in 25 mL of water.
CONCENTRATION:
The concentration of a solution is related to the amount of a substance and its volume. The concentration of a solution is directly proportional to the amount of a substance but inversely proportional to the volume. This means that the concentration increases with an increasing amount of substance but decreases with an increasing volume. According to this question, 15g of sugar is the highest amount and it dissolves in 25mL of water, which is the lowest volume. Therefore, it is the most concentrated sugar solution.Learn more at: https://brainly.com/question/202460?referrer=searchResults
write the properties of first group of element
Answers
Alkali metals are grey solids with shiny silvery surfaces when freshly cut.
These surfaces turn dull when exposed to air.
This is because alkali metals are very reactive. They react rapidly with oxygen and water vapour in the air when exposed.
Answer:
hydrogen
Explanation:
1
H
1
hydrogen
magnesium
What functional group is always found in alkaloids (such as caffeine, nicotine e pyreale) 1.amide 2. Acid 3. Amine 4. Ether 5. Ester
Answers
Answer:
3.Amine
Explanation:
The functional group that is always found in alkaloids (such as caffeine, nicotine, and pyreale) is the amine functional group.
However, some alkaloids may also contain other functional groups, such as amides.
The functional group that is always found in alkaloids (such as caffeine, nicotine, and pyrethroids) is the amine group. So, the correct answer is option 3. Amine. Alkaloids are a class of naturally occurring organic compounds that mostly contain basic nitrogen atoms. The amine functional group consists of a nitrogen atom bonded to one or more alkyl or aryl groups. Amides, on the other hand, are a different functional group that involves a nitrogen atom bonded to a carbonyl group.
Visit here to learn more about alkaloids:
brainly.com/question/14298126
#SPJ11
Some of the energy given off by the sun is in the form of _________ and ________energy
Answers
Answer:
All of the energy from the Sun that reaches the Earth arrives as solar radiation, part of a large collection of energy called the electromagnetic radiation spectrum. Solar radiation includes visible light, ultraviolet light, infrared, radio waves, X-rays, and gamma rays.
Explanation:
Marco put a pot of water on to boil eggs. after a few minutes all the water was This is an example of _____.
Multiple choice question.
A)
condensation
B)
deposition
C)
sublimation
D)
vaporization
Answers
Answer:
D) vaporizationExplanation:
Marco put a pot of water on to boil eggs. after a few minutes all the water was dry
This is an example of vaporization.hopefully this helps you!
Radiation is when heat is transferred through
A.
the
air
B. the object
O
C. the sun
D. the liquid
Answers
Answer:
I think its C
Explanation:
because it travels from the sun to earth
A 325 0 g piece of gold at 427 degree C is dropped into 200.OmL of water at 22 0 degree C. Calculate the final temperature of the mixture. Specific Heat of gold
Answers
The final temperature of the mixture is 218.1°C.
The Specific Heat of gold 0.0123 J.
What is specific heat ?Specific heat is known to be the amount of energy required to raise the temperature of a unit mass of a substance by 1 degree. It is a physical property of matter, usually expressed in units of Joules/Kilograms/Kelvin (J/kgK).
Mass of gold = 325 g
Heat absorbed by water = mass of gold x specific heat x temperature change
325 x 0.128 x 405
Heat absorbed by water = 154,400 J
Heat capacity of water = mass of water x specific heat
200 x 4.184
Heat capacity of water = 836.8 J/degree Celsius
Final temperature = (heat absorbed by water ÷ heat capacity of water) + Initial temperature
Final temperature = (154400 ÷ 836.8) + 22
Final temperature = 218.1°C
Q = mcΔT
836.8 J/°C = 325 × c × (427°C - 218.1°C)
836.8 J/°C = 325 × c × 208.9
c = 836.8/(325 × 208.9)
c = 836.8/67,892.5
c = 0.0123 J
To learn more specific heat
https://brainly.com/question/21041726
#SPJ4
A chemist heats a fixed amount of gas in a sealed glass container. Which law is the chemist most likely investigating?
Answers
The law which this chemist is most likely investigating heating a fixed amount of gas in a sealed glass container is: Gay Lussac’s law.
Gay Lussac's law states that when the volume of an ideal amount of gas is held (kept) constant, the pressure of the gas is directly proportional to the absolute (Kelvin) temperature of the gas.
Mathematically, Gay Lussac's law is calculated by using the formula;
\(PT = K\\\\\frac{P_1}{T_1} = \frac{P_2}{T_2}\)
Where:
\(P_1\) is the original pressure.\(P_2\) is the final pressure.\(T_1\) is the original temperature.\(T_2\) is the final temperature.In conclusion, the chemist is most likely investigating how changes in temperature affect the pressure of the gas by heating a fixed amount in a sealed glass container in accordance with Gay Lussac's law.
Read more: https://brainly.com/question/24928274
Gases Unit Test Honors Chemistry A
1) B. The volume increases to twice its original value.
2) A. volume and temperature directly proportional
pressure and volume inversely proportional
pressure and temperature directly proportional
3) D. Gay-Lussac’s law, by seeing how changes in temperature affect the pressure of the gas
4) B. V1P1/T1=V2P2/T2
5) A. It is a straight line with a positive slope showing that an increase in temperature results in an increase in volume.
6) D. keeping the pressure constant and increasing the temperature
7) A. When temperature is held constant and volume increases, the pressure increases.
8) D. pressure, volume, temperature, number of moles
9) A. volume
10) A. 7.10 L/mol
11) A. 0.105 mol
12) B. 27 g/mol
13) B. the temperature increasing by a factor of 2
14) brainly.com/question/24915187
15) brainly.com/question/24544023
16)brainly.com/question/24544061
17) brainly.com/question/24598785
ultraviolet, visible, and infrared light are all examples of __ radiation, which has the properties of both particles and __.
Answers
Ultraviolet, visible, and infrared light are all examples of non-ionizing radiation, which has the properties of both particles and waves.
Non-ionizing radiation is defined as a type of low-energy radiation that does not have enough energy to remove an electron (negative particle) from an atom or molecule. Generally, non-ionizing radiation includes visible, infrared, and ultraviolet light, microwaves, radio waves, and radiofrequency energy from cell phones.
Wave is defined as a disturbance or variation that transfers energy progressively from point to point in a medium and that may take the form of an elastic deformation or of a variation of pressure, electric or magnetic intensity, electric potential, or temperature.
Learn more about non-ionizing radiations from the link given below.
https://brainly.com/question/1504254
#SPJ4
the side chain of an amino acid differentiates one amino acid from another. True or False?
Answers
True, the side chain of an amino acid differentiates one amino acid from another.
What is an amino acid?Amino acids are the building blocks of protein, which is a macronutrient essential for the growth and repair of body tissues, among other things.
The side chain of an amino acid distinguishes it from other amino acids. Amino acids are organic compounds that have two functional groups: an amino group (-NH2) and a carboxyl group (-COOH). They can be divided into two categories: non-essential amino acids, which are made by the body, and essential amino acids, which must be obtained from food.
Learn more about amino acid: https://brainly.com/question/21327676
#SPJ11
which of the following compounds has a vibration that is infrared inactive?
H2S 2-butyne ethene 1-butyne Nz O2
Answers
Among the given compounds, nitrogen gas (N2) has a vibration that is infrared inactive. The other compounds, H2S, 2-butyne, ethene, and 1-butyne, have vibrations that are infrared active.
Infrared spectroscopy is a technique used to identify functional groups and molecular vibrations in a compound. When a molecule absorbs infrared radiation, it undergoes a change in vibrational energy levels.
To be infrared active, a molecule must have a change in dipole moment during a vibration. This occurs when there is a net movement of charge within the molecule. Among the given compounds, H2S, 2-butyne, ethene, and 1-butyne all have polar bonds and undergo changes in dipole moment during vibrations. Therefore, they are infrared active.
On the other hand, nitrogen gas (N2) consists of a homonuclear diatomic molecule with a nonpolar bond. Since there is no change in dipole moment during the vibrations of N2, it is infrared inactive.
To learn more about spectroscopy click here: brainly.com/question/13265448
#SPJ11
describe the manufacture of ethanol from hexane?
Answers
Answer:
This page looks at the manufacture of alcohols by the direct hydration of alkenes, concentrating mainly on the hydration of ethene to make ethanol.
The steps in the ethanol production process include milling the corn to meal, liquefying the meal by adding water and cooking, breaking down starch into sugar, using yeast to ferment the sugar to ethanol, distilling the ethanol by boiling off and condensing it by removing residual water and finally denaturing so that...
When a person runs, how is
the energy transformed?
A. The chemical energy in their food is
transformed into sound and thermal energy.
B. The mechanical energy in their food is
transformed into electromagnetic and
thermal energy.
C. The chemical energy in their food is
transformed into mechanical and thermal
energy.
Answers
The energy can neither be created nor be destroyed. But it can be converted from one form to another. When a person runs, The chemical energy in their food is transformed into mechanical and thermal energy. The correct option is C.
What is mechanical energy?The mechanical energy of an object is the sum of the potential and kinetic energies which is used to do a particular work. It represents the energy of an object due to its motion or position or both.
The body takes chemical energy in the form of food. when a person runs this chemical energy is utilized by our body and converted into kinetic energy (Mechanical). The body get heated by running which then releases thermal energy.
Thus the correct option is C.
To know more about mechanical energy, visit;
https://brainly.com/question/28928306
#SPJ9
Compare these two pictures to the graph above. In which of these situations did they use a catalyst (the left or right)? Why? In which of these two graphs will a reaction actually take place?
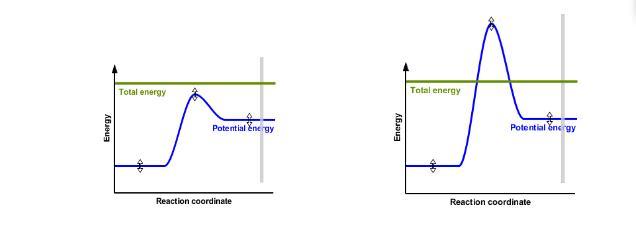
Answers
Answer:
Explanation:
The role of enzymes in a chemical reaction is to : Lower the activation energy while increasing the rate of reaction
The graph shown on the left is an uncatalyzed chemical reaction while the graph on the right is a catalyzed chemical reaction.
Catalyzed chemical reactions have lower activation energy and a faster rate of reaction while uncatalyzed chemical reaction has a higher activation energy and a slower reaction rate. because catalyst increases the rate of a chemical reaction by lowering its activation energy.
Hence we can conclude that The role of enzymes in a chemical reaction is to : Lower the activation energy while increasing the rate of reaction
Question 4 (1 point)
How many oxygens are present on the reactants side of this equation?
KCIO3 --> KCI + O₂
01
5
3
2
Answers
Oxygens are present on the reactants side of this equation is 3
Reactant is a substance that enters into and is altered in the course of a chemical reaction and the substance to the left of the arrow in a chemical equation are called reactant and a reactant is a substances that is present at the start of a chemical reaction and the substances to the right arrow are called product and a product is a substances that is present at end of chemical reaction
Here given reaction is
KCIO₃ → KCI + O₂
Here 3 oxygen atom are seen in the reactant so 3 oxygen atom are in the reactant side in the equation
Know more about oxygens
https://brainly.com/question/22394338
#SPJ1
List and describe two differences between pure substances and mixtures. Check all that apply. Check all that apply. The composition of a pure substance depends on the source, but the composition of a mixture doesn't depend on the source. The composition of a pure substance is always the same, regardless of the source, but the composition of a mixture can vary. The state of a pure substance is always the same, but the state of a mixture can vary. Mixtures can be separated into their components only by chemical changes; some pure substances can be separated into components by physical change. Mixtures can be separated into their components by physical changes; some pure substances can be separated into components by chemical change.
Answers
Answer:
The composition of a pure substance is always the same, regardless of the source, but the composition of a mixture can vary.
Mixtures can be separated into their components by physical changes; some pure substances can be separated into components by chemical change.
Explanation:
A chemically pure substance has a well defined and constant composition. The composition of a chemically pure substance remains the same irrespective of its source. Also, the components of a chemically pure substance may be separated by chemical changes.
Mixtures have a variable composition depending on their respective sources. The composition of a mixture varies with the source of the mixture and mixtures are separated by physical processes.
A 360. g iron rod is placed into 750.0 g of water at 22.5°C. The water temperature rises to 46.7°C. What was the initial temperature of the iron rod?
Answers
Answer: The initial temperature of the iron was \(515^0C\)
Explanation:
\(heat_{absorbed}=heat_{released}\)
As we know that,
\(Q=m\times c\times \Delta T=m\times c\times (T_{final}-T_{initial})\)
\(m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]\) .................(1)
where,
q = heat absorbed or released
\(m_1\) = mass of iron = 360 g
\(m_2\) = mass of water = 750 g
\(T_{final}\) = final temperature = \(46.7^0C\)
\(T_1\) = temperature of iron = ?
\(T_2\) = temperature of water = \(22.5^oC\)
\(c_1\) = specific heat of iron = \(0.450J/g^0C\)
\(c_2\) = specific heat of water= \(4.184J/g^0C\)
Now put all the given values in equation (1), we get
\(-360\times 0.450\times (46.7-x)=[750\times 4.184\times (46.7-22.5)]\)
\(T_i=515^0C\)
Therefore, the initial temperature of the iron was \(515^0C\)
Which of the following is the best definition of ethics?
A. The maintenance of a stable internal environment
B. Response to external stimuli
C. A set of guidelines for fair conduct during an experiment
D. A multistep process whose last step controls what happens in an
earlier step
Answers
Answer:
C
Explanation:
I believe the answer is C.
How do we solve this question? I found B answer key says A

Answers
First, we write the reaction and balance it:
HNO2 (aq) + NH3 (aq) = NH4+ + NO2- (Balanced)
Data:
50 mL of 0.2 M HNO2
50 mL of 0.2 M NH3
In total, we have 100 mL, therefore, this solution between HNO2 and NH3 will be diluted in half. I mean: The concentration of HNO2 and NH3 will be 0.10 M
HNO2 (aq) + NH3 (aq) = NH4+ + NO2-
Initial 0.10 M 0.10 M 0 0
reacts -x -x +x +x
Equilibrium 0.10-x 0.10-x +x +x
Now, we write Kc:
\(\begin{gathered} Kc\text{ = }\frac{\lbrack NH4+\rbrack\lbrack NO2-\rbrack}{\lbrack HNO2\rbrack\lbrack NH3\rbrack}=\frac{x^2}{(0.10-x)^2} \\ 1x10^6=\frac{x^2}{(0.10-x)^2} \\ \sqrt{1x10^6}=\text{ }\lvert{\frac{x}{(0.10-x)}}\rvert \\ We\text{ get 2 values here:} \\ 1)+1000=\frac{x}{(0.10-x)} \\ and \\ 2)-1000\text{ = }\frac{x}{(0.10-x)} \end{gathered}\)Values of x:
For 1) x = 0.0999
For 2)x = 0.1001
We choose number 1) x = 0.0999
Number 2 gives us a value higher than the initial values of concentration
Therefore, concentration in equilibrium of NH3 = 0.10-x =0.10 - 0.0999 = 0.00010M
Answer: A. 0.00010M
Which of the following are medical applications of radiation? Select all that apply.
Answers
The following which are medical applications of radiation include the following below:
Cancer therapyGenetic engineering.Detecting thyroid malfunction.What is Radiation?This is referred to as the transmission of energy through space and it usually occurs at the speed of light thereby making it a very fast method of heat transfer when compared to the others. Radiation are used for various purposes such as immigration for searching luggage etc.
It is also used for medical reasons such as cancer therapy as the radiation help in destroying the cancerous cells thereby making it easy for their removal from the body system. It is also used for genetic engineering in the creation of new types of substances through the modification of nucleic acids such as DNA etc.
Read more about Radiation here https://brainly.com/question/24469662
#SPJ1
when magnesium reacts with chlorine, the chlorine atom gain electrons. what happens to chlorine in this reaction?
a. it is oxidized
b. it is synthesized
c. it is decomposed
d. it is reduced
Answers
Answer:
D
Explanation:
Reduction refers to gain in electrons. Hence it is D
Oxidation occurs when the element loses electrons. Hence A is wrong
How many phosphorus atoms are contained in 158 kg of phosphorus
Answers
Step 1 - First, we need to transform kg into g. We need to multiply it by 1000:
158 kg = 158,000 g of phosphorus
Step 2 - Now we need to know the molar mass of phosphorus. Look for it at the periodic table. It is 30.97 g/mol.
Step 3 - We transform grams into moles:
30.97 g ---- 1 mol
158,000 g ---- x mol
x = 5,101.7 moles of phosphorus
Step 4 - We transform moles into atoms using Avogrado's constant:
6.022 x 10^23 ---- 1 mol
x ---- 5,101.7 moles of phosphorus
x = 3.072 x 10^27 atoms
If you want to calculate it in another way:
158000/30.97 x 6.022 x 10^23 = 3.072 x 10^27 atoms
Answer: There are 3.072 x 10^27 atoms of phosphorus
Which of the following best describes an exothermic reaction?
O A chemical reaction that absorbs heat from its environment
O A chemical reaction that releases heat into its environment
O
A chemical reaction that absorbs and releases heat
Answers
Answer: A chemical reaction that releases heat into its environment
Explanation:
the exothermic process is a process or reaction that releases energy, (usually in the form of heat but also in light, electricity, or sound) into its environment
ex:
think of ice freezing, to change from a liquid to a solid it must release heat to lower it’s temperature to reach its freezing point
at constant temperature, four grams of hydrogen gas is added to a 12.0 g sample of nitrogen gas in a fixed volume container with an initial pressure of 1.00 atm. if the container is built to shatter at pressures over 3.5 atm, will the container remain intact?
Answers
If the container is built to shatter at pressures over 3.5 atm, the container will remain intact.
At constant temperature, when four grams of hydrogen gas is added to a 12.0 g sample of nitrogen gas in a fixed volume container with an initial pressure of 1.00 atm, we can determine if the container will remain intact using the formula for partial pressures:
P_total = P1 + P2
First, we need to find the moles of each gas. Hydrogen has a molar mass of 2 g/mol, and nitrogen has a molar mass of 28 g/mol.
Moles of H₂ = 4 g / 2 g/mol = 2 mol
Moles of N₂ = 12 g / 28 g/mol = 0.429 mol
Now we can find the mole fractions of each gas:
Mole fraction of H₂ = 2 mol / (2 mol + 0.429 mol) = 0.823
Mole fraction of N₂ = 0.429 mol / (2 mol + 0.429 mol) = 0.177
Next, we can find the partial pressures:
P_H₂ = Mole fraction of H₂ * Initial pressure = 0.823 * 1.00 atm = 0.823 atm
P_N₂ = Mole fraction of N₂ * Initial pressure = 0.177 * 1.00 atm = 0.177 atm
Finally, we find the total pressure:
P_total = P_H₂ + P_N₂ = 0.823 atm + 0.177 atm = 1.00 atm
Since the total pressure is 1.00 atm, which is below the container's shatter limit of 3.5 atm, the container will remain intact.
Learn more about pressure at https://brainly.com/question/28012687
#SPJ11
All the simple machines make work easier to do by changing the _____ or _____ of a force. A. size; type B. work; type C. size; direction D. type; direction
Answers
Answer:
C. size; direction
Explanation:
By definition, a machine is referred to any device that makes work easier. It takes force to do work, hence, work refers to the application of force over a particular distance. A machine aims at making the work easy by changing how it is done. Simple machines, which include: levers, pulleys, inclined planes etc. all carry out the same thing, which is to make work easier, by changing the size/magnitude and direction of the applied force.
A simple machine tends to change the size of the inputted force by increasing it over a shorter distance. The machine increases the force applied better than it can be done manually e.g. a plier and nutcracker increases/changes the applied force better than it can be done with bare hands.
Also, a simple machine can achieve making work easier by changing the direction at which the force is applied. The machine applies the force on the object in an opposite direction or contrary to the way it was manually applied.