Predict the major organic product of the following reaction.
Answers
Answer:What is minor and major?
minor is a smaller scale than major, and may be played on lower strings while major is played on higher strings. In the music world, minor scales are often used to create melodies and chord progressions.
Related Questions
Explain what lattice energy is and how it affects
the properties of ionic compounds. Describe the
general properties of ionic compounds.
Answers
Answer:
Explanation:
Lattice energy is a way to measure the bond strength in ionic compounds. Ionic compounds tend to have high melting and boiling points. Ionic compounds tend to be hard. Ionic compounds have a range of solubilities.
calculate the number of mole and molecule in 10g water
Answers
Answer:
1 mole of water weighs 18g. 10/18 moles = 10/18 * 6 * 10^23 number of molecules = 3.33 * 10^23 molecules of water. Each molecule of water has 3 atoms, so the total number of atoms in 10g of water is 3*3.33*10^23 = 10^24.
Explanation:
Which of the following terms refers to the area immediately around the eye
Answers
Answer:
the eye socket
Explanation:
the area around the eye is called the eye socket or the eye orbit.
Consider the reaction
N2 + 3 H2 → 2 NH3 .
How much NH3 can be produced from the reaction of 74.2 g of N2 and 14.0 moles of H2?
Answers
The mass of ammonia that can be produced from the reaction of 74.2 g of N₂ and 14.0 moles of H₂ is 90.1 g.
What is the mole ratio of the reaction for the production of ammonia?The mole ratio of the reaction for the production of ammonia is obtained from the balanced equation of the reaction as follows:
Equation of the reaction: N₂ (g) + 3 H₂ (g) → 2 NH₃ (g)
The mole ratio of the reactants nitrogen and hydrogen is 1 : 3
Moles of nitrogen in 74.2 g of nitrogen = 74.2/28
Moles of nitrogen in 74.2 g of nitrogen = 2.65 moles
Moles of hydrogen present = 14.0 moles
The limiting reactant is nitrogen
Mole ratio of nitrogen to ammonia is 1 : 2
Mass of ammonia produced = 2.65 * 2 * 17 g
Mass of ammonia produced = 90.1 g
Learn more about mole ratio at: https://brainly.com/question/19099163
#SPJ1
Why do scientists clone banana plants, but not humans?
A.
Scientists have only cloned plants, not animals.
B.
Cloning a human would do little to benefit scientific research.
C.
All scientists agree that cloning humans is scientifically impossible.
D.
There are legal and ethical restrictions regarding human cloning.
Answers
Cloning is used for to create duplicate of other cell or organism. Because there are legal and ethical restrictions regarding human cloning. So, option D is correct.
Why scientist clone banana plants ?The process in which replication occurs to produce another cell, tissue or organism is called as cloning. It increase the disease resistance and yield.
Banana cloning allows them to be a crop provides high yields, with or without pollination and seed formation. But before domestication, most banana varieties produces large number of seeds in the middle of the fruit.
Scientists clone banana plants it gives genetic modification for the lack of traditional breeding opportunities. It is an effective way to develop bananas yield.
Hence, option D is correct.
To learn more about cloning of banana refer the link below;
https://brainly.com/question/14266296
#SPJ2
Match each label below with the appropriate term. Note there may be more than one correct answer.
H-0:
nonbonding electrons
sigma bond
represents two electrons
Answers
Answer:
sigma bond
represents two electrons
Explanation:
The bond between hydrogen and oxygen is sigma bond because the bond between hydrogen and oxygen is covalent means sharing of electron takes place between two atoms and we know that sigma bond is also occurs in a covalent bond in which overlapping of atomic orbitals or hybrid orbitals along the bond axis occurs. Both also represent two electrons because both atoms share one electron each so we can say that the two atoms represents two electrons.
five grams of sugar are added to a 45-gram serving of a breakfast cereal that is 10% sugar. what is the percent concentration of sugar in the resulting mixture?
Answers
The resulting mixture will have a sugar concentration of 22.22% when 5g of sugar is added to a 45g of serving which is 10% sugar.
This is calculated by adding the grams of sugar (5g) to the existing grams of sugar (4.5g) and dividing by the total grams of cereal (45g). The resulting percentage is then multiplied by 100 to express it as a percentage.
5+4.5=9.5
9.5/45×100=21.11
To put this into perspective, the original 10% sugar concentration translates to 4.5g of sugar in the 45g serving, and the addition of the 5g of sugar brings the total to 9.5g of sugar.
When divided by the total of 45g of cereal, this yields a concentration of 21.11%, which is then multiplied by 100 to give the final percentage of 22.22%.
Learn more about concentration:
https://brainly.com/question/24147934
#SPJ4
To use the gas law constant R = 0. 0821, the unit for temperature should be Kelvin and the unit for volume should be milliliters.
true or false
Answers
the given statement was false cause when the gas law constant R = 0. 0821 was used the temperature should be in K and the volume should be in liters.
define gas law constant ?
In chemistry and physics, "R" is the symbol for the gas constant, molar gas constant, ideal gas constant, or universal gas constant. In numerous equations, it is a proportionality factor that connects energy and temperature scales.
Chemistry's Gas Constant
The gas constant is also known as the ideal gas constant and the universal gas constant in chemistry.
The Boltzmann constant has a molar equivalent.
The gas constant has a SI value of 8.31446261815324 JK1mol1. The number is usually rounded to 8.314.
the given statement was false cause when the gas law constant R = 0. 0821 was used the temperature should be in K and the volume should be in liters.
To learn more about gas constant follow the given link: https://brainly.com/question/29034664
#SPJ1
a btu is defined as the amount of energy required to raise the temperature of one lb of water by one degree celsius t or f
Answers
British thermal units (Btu) are a unit used to measure the amount of heat in fuels or other energy sources.
The amount of heat needed to raise a pound of liquid water's temperature by one degree Fahrenheit at the point where water has its highest density (approximately 39 degrees Fahrenheit).
Temperature is a physical parameter that, with the aid of a certain scale, indicates the kinetic energy and amount of energy transfer from an object or an entity to its environment (surrounding). Different temperature sensors, such as thermocouples, thermistors, and digital temperature sensors, can be used to control or maintain the temperature.
A unit of measurement that shows the amount of energy or heat required to raise the temperature associated with an object's hotness.
Learn more about British thermal units:
brainly.com/question/1224318
#SPJ4
You will be given 4.6x10^3 mg of Sodium Chloride, how many atoms of NaCl do I have?
Answers
The total number of molecules that are present in 4.6×\(10^{3}\) mg of NaCl is 0.47×\(10^{23}\)
Atomic masses:
Na=23 , Cl=35.5
The molar mass of NaCl=58.5
1gm of NaCl contains=1/58.5 moles of NaCl
And,
1gm of NaCl contains=1/58.5×6.022×\(10^{23}\) molecules of NaCl
4.6x\(10^{3}\) mg =(4.6x\(10^{3}\)x\(10^{-3}\) gm) of NaCl contains= \(\frac{1}{58.5}\) ×6.022×\(10^{23}\)×4.6
=0.017×27.70×\(10^{23}\)
=0.47×\(10^{23}\)
Therefore the total number of molecules present in 4.6×\(10^{3}\) mg NaCl is 0.47×\(10^{23}\)
Learn more about molar mass:
https://brainly.com/question/28447857
why does the d block start in the fourth row of the periodic table
Answers
Answer:
Why does D-block start on the fourth row of the periodic table?
Explanation:
The require more energy to reach 3d than 4s, and they fill up 4p before 4d, and so forth. ... The energy is lower for 6s than 4f, and 4f and 5f are lower in energy than the final D level.
Magnesium (Mg) has nine electrons. Which of the following shows the correct electron configuration for an atom of Mg?
2, 2, 5
7, 2
1, 8
2, 7
brailyist please help me
Answers
Answer:
2,7
Explanation:
Two in the first shell and 7 in the outer and last shell.
HOPE THIS HELPED
why does light striking a metal surface eject only electrons and protons
Answers
How many grams of water are produced by the combustion of 32.0g of ch4?
CH4+202–>2H2+CO2
Show your work
Answers
The combustion reaction must involve oxygen as one of the reactant. The grams of water produced by the combustion of 32.0g of CH₄ is 72 g.
What is combustion reaction?The combustion can be defined as a chemical process or reaction between the hydrocarbon and oxygen. When they react together, heat and light energy is released. The burning of wood is an example.
Here the reaction is:
CH₄ +2O₂ → 2H₂O +CO₂
Combustion of one mole of CH₄ gives 2 moles of water (36 g).
Then combustion of 32 g of CH₄ gives:
36 g H₂O/16 g CH₄ × 32 g CH₄ = 72 g water
Thus the grams of water produced is 72 g.
To know more about combustion, visit;
https://brainly.com/question/14335621
#SPJ1
The half life for uranium-235 is 7.0x10 8years. a. How many half-lives did the sample go through at the end of 2.8x10 9years? b. How much of a 0.74mg sample of uranium-235 will remain after 2.8x10 9years?
Answers
Answer:
A 4 half-life
B. 0.05 mg
Explanation:
A. Determination of the number of half-lives after 2.8×10⁹ years.
From the question given above,
7×10⁸ years = 1 half life
Therefore
2.8×10⁹ years = 2.8×10⁹ years × 1 half life / 7×10⁸ years
2.8×10⁹ years = 4 half life
Thus, the sample went through 4 half-lives at the end of 2.8×10⁹ years.
B. Determination of the amount of the sample remaining after 2.8×10⁹ years.
Original amount (N₀) = 0.74 mg
half life (t½) = 7×10⁸ years
Time (t) = 2.8×10⁹ years
Amount remaining (N) =?
Next, we shall determine the rate of disintegration. This can be obtained as follow:
half life (t½) = 7×10⁸ years
Decay constant (K) =?
K = 0.693 / t½
K = 0.693 / 7×10⁸
K = 9.9×10¯¹⁰ /year
Finally, we shall determine the amount remaining as follow:
Original amount (N₀) = 0.74 mg
Time (t) = 2.8×10⁹ years
Decay constant (K) = 9.9×10¯¹⁰ /year
Amount remaining (N) =?
Log (N₀/N) = kt / 2.303
Log (0.74/N) = 9.9×10¯¹⁰×2.8×10⁹ /2.303
Log (0.74/N) = 2.772 / 2.303
Log (0.74/N) = 1.2036
Take the antilog of 1.2036
0.74/N = antilog (1.2036)
0.74 / N = 15.98
Cross multiply
0.74 = N × 15.98
Divide both side by 15.98
N = 0.74 / 15.98
N = 0.05 mg
Thus, 0.05 mg of the sample will remain after 2.8×10⁹ years
The amount of uranium sample remained after 4 cycles in \(\rm 2.8\;\times\;10^9\) years has been 0.04625 mg.
The half-life can be described as the time required by the element to reduce to its half concentration from the initial concentration.
A. The number of half-life cycles can be calculated as:
\(\rm 7.0\;\times\;10^8\) = 1 cycle
\(\rm 2.8\;\times\;10^9\) = \(\rm \dfrac{1}{\rm 7.0\;\times\;10^8}\;\times\;2.8\;\times\;10^9\)
= 4 cycles.
The number of half-life cycles after \(\rm 2.8\;\times\;10^9\) years are 4 cycles.
B. The amount of sample remained can be calculated as:
Sample remained = Initial sample \(\rm \times\;\dfrac{1}{2}^\frac{time}{Half-life}\)
Sample remained = 0.74 mg \(\rm \times\;\dfrac{1}{2}^\frac{2.8\;\times\;10^9}{7.0\;\times\;10^8}\)
Smaple remianed = 0.74 \(\rm \times\;\dfrac{1}{2}^4\)
Sample remained = 0.74 \(\times\) 0.0625 mg
Sample remained = 0.04625 mg.
The amount of uranium sample remained after 4 cycles in \(\rm 2.8\;\times\;10^9\) years has been 0.04625 mg.
For more information about the half-life, refer to the link:
https://brainly.com/question/24710827
What is the importance of a special habitat?
Answers
ma2b2 is a molecule where m is the central atom bonded to 2 atoms of a and 2 atoms of b. the fact that it exists in only one form (no isomers) suggests its geometry is select one: a. octahedral b. square planar c. tetrahedral d. trigonal planar
Answers
hello
the answer to the question is C) tetrahedral
Why is it impossible for an element to have an atomic number of 110.5?
a. atoms of an element have the same whole number of protons and neutrons
b. atoms of an element all have the same whole number of protons
c. exactly half of the isotopes would need an atomic number of 110 and half would need an atomic number of 111 which is very unlikely
d. atoms with atomic numbers greater than 100 are unstable
Answers
Answer: B
Explanation: The atomic number is based only on the number of protons in the nucleus of an atom. Since this is a count of whole numbers, it cannot be a decimal. It's either element 110 or 111, not 110.5.
Answer:
B
A
C
A
C
These should be the answers for your test. If you have any questions, please DM me
Calculate how many molecules are in 25.1 grams of N2.
answer choices:
1. 1.08 x 10^24 molecules N2
2. 6.02 x 10^23 molecules N2
3. 6.31 x 10^22 molecules N2
4. 5.39 x 10^23 molecules N2
Answers
which species in the chemical reaction agno3 nai → agi nano3 are the products?
Answers
In the chemical reaction AgNO3 + NaI → AgI + NaNO3, the products are AgI and NaNO3.
This is a double displacement reaction where the two reactants switch their ions to form two new compounds. AgNO3 is silver nitrate and NaI is sodium iodide. When they are mixed, the silver ion (Ag+) from AgNO3 combines with the iodide ion (I-) from NaI to form silver iodide (AgI).
At the same time, the nitrate ion (NO3-) from AgNO3 combines with the sodium ion (Na+) from NaI to form sodium nitrate (NaNO3). AgI is a yellow solid that is sparingly soluble in water, while NaNO3 is a white solid that is highly soluble in water.
Learn more about reactant at https://brainly.com/question/11726191
#SPJ11
HELP ASAP
When a carbon atom joins chemically with an oxygen atom, it makes carbon monoxide. What is the term for carbon monoxide? O A. A mixture B. An element O C. An alloy D. A molecule
Answers
D- A molecule
Explanation:
(Apex)
is Si2Br6 an ionic, polar covalent, or non-polar covalent bond?
Answers
Answer:
non-polar covalent bond
The covalent bonds form silicon dibromide, or \(Si_2Br_6\). Instead of being an ionic compound, it is a covalent one.
The sharing of electrons between the silicon (Si) and bromine (Br) atoms in \(Si_2Br_6\) provides the basis for their bonding. Covalent bonds are formed when atoms share electrons to form a stable electron configuration. In this example a total of twelve electrons are shared between each of the silicon atoms and six of the bromine atoms. By filling their valence electron shells, this electron sharing enables silicon and bromine to have more stable structures.
Learn more about electrons, here:
https://brainly.com/question/12001116
#SPJ6
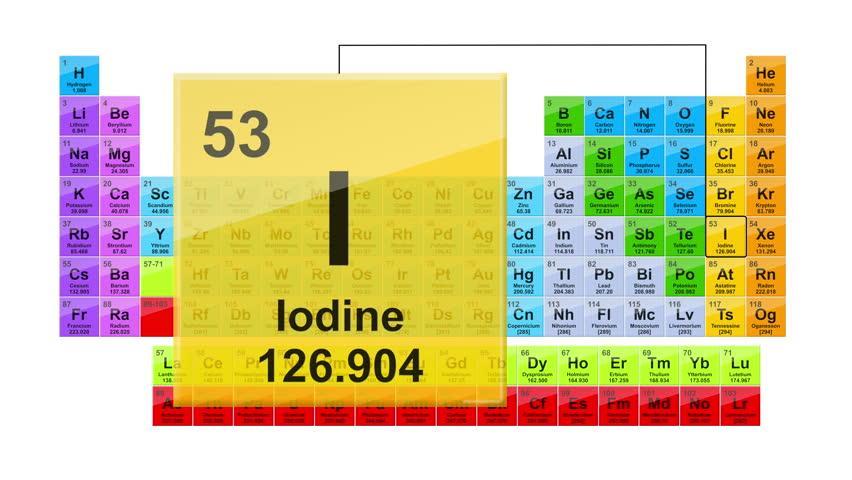
Which element is in Group 17 and has more than 50 protons but less than 75 protons?
Answers
Answer:
Iodine
Explanation:
Iodine is in group 17 on the periodic table (first picture attached). The second picture shows you in the top left-hand corner of Iodine's little square, there is the number 53. This is the atomic number, it is also the number of protons and electrons in an element.
Hope this helped :)

Which contributes most to the hybrid?
a. the structures with the positive charge on carbon.
b. all contribute equally.
c. the structure with the positive charge on sulfur.
Answers
Option a, which involves a positive charge on carbon, has the potential to make a greater contribution to the overall hybrid structure than option c, which features positive charges on sulfur.
a. The structure with the positive charge on carbon: This option indicates that there is a positive charge on a carbon atom. Carbon is less electronegative than sulfur, meaning it has a lower affinity for electrons. As a result, a positive charge on carbon is more favorable and stable compared to a positive charge on sulfur. Carbon can accommodate the positive charge by sharing its electrons with adjacent atoms, forming stable carbon cations.
b. All contribute equally: This statement suggests that all the resonance structures provided contribute equally to the hybrid. However, in reality, resonance structures can have different contributions based on factors such as the location and stability of charges. So, this answer is not accurate.
c. The structure with the positive charge on sulfur: This structure indicates that there is a positive charge on a sulfur atom. In organic chemistry, a positive charge on sulfur is less stable than a positive charge on carbon. Sulfur, is more electronegative than carbon. It has a higher affinity for electrons and prefers to maintain a full octet rather than carrying a positive charge. Positive charges on sulfur are less stable and tend to be more reactive and less common in organic compounds.
Learn more about electronegativity:
https://brainly.com/question/18258838
#SPJ11
A gene or trait that appears or expresses itself over a recessive trait is called the anstwer
Answers
How many moles of NaCl are present in 23.5 grams of NaCl?
Answers
Answer:
0.342 mol
Explanation:
Molar mass of
NaCl = 58.4 g/mol
Number of moles in
20.0 g NaCl
is
20.0
g
58.4
g
/mol
=
0.342 mol
What is the balance equation for
_ Ba + _ HNO3 —> _ H2 + _ Ba(NP3)2
_ H3PO4 + _ NaOH —> _ H2O + _ Na3PO4
Answers
1st one
2_2_2
2nd one
3_1_1_3
i looked it up
How did the pressure in the left intrapleural cavity change when the valve was opened?
O The pressure in the intrapleural cavity equalized with the atmospheric pressure.
O It went from a positive number to a negative number.
O It went from a positive number to a negative number and the pressure in the intrapleural cavity equalized with the atmospheric pressure.
O It went from a negative number to zero and the pressure in the intrapleural cavity equalized with the atmospheric pressure.
O It went from a negative number to zero.
Answers
The pressure in the left intrapleural cavity changes when the valve was opened as : It went from negative number to zero and the pressure in intrapleural cavity equalized with the atmospheric pressure.
What is meant by intrapleural cavity?The pressure inside the pleural cavity is called as intrapleural pressure. It is characterized as negative pressure when the pressure inside the pleural cavity is typically only a little bit lower than the ambient pressure.
The external intercostal muscles and diaphragm contract during inspiration, expanding the thoracic cavity. As a result, the intrapleural pressure decreases, increasing the transpulmonary pressure and expanding the lungs.
To know more about intrapleural cavity, refer
https://brainly.com/question/9209660
#SPJ4
Problem: Co3+ | Co2+ and Ni2+ | NiAnode?Cathode?(You need to use Reference Table B-16.)a. Co2+b. can't answerc. Ni2+d. Nie. Co3+

Answers
Answer:
- Anode: Co3+ | Co2+
- Cathode: Ni | Ni2+
Explanation:
The anode is where oxidation reaction occurs, and the cathode is where reduction reaction occurs.
From the table of reduction potencials, we find that:
- Co reaction:
\(\begin{gathered} Co^{3+}+2e^-\rightarrow Co^{2+} \\ E=1.81\text{ }V \end{gathered}\)- Ni reaction:
\(\begin{gathered} Ni\rightarrow Ni^{2+}+2e^- \\ E=-0.250\text{ V} \end{gathered}\)Now, to find out which one is the anode and which one is the cathode, it is necessary to compare the reduction potencials.
The reaction of Ni have negative potentials, so Ni will be the anode and Co will be the cathode.
in any organic redox reaction, you can recognize the reduced and oxidized organic molecules by tracking the charges between products and reactants. reduction corresponds to
Answers
Hi! In an organic redox reaction, you can indeed identify the reduced and oxidized organic molecules by monitoring the charges between the reactants and products. In a redox reaction, there is a transfer of electrons between molecules, leading to a change in their oxidation states. To understand this better, let's break down the two processes involved in a redox reaction: reduction and oxidation.
Reduction corresponds to a gain of electrons by a molecule, causing a decrease in its oxidation state. This means that the reduced molecule becomes more negatively charged or less positively charged. In organic reactions, reduction often involves the addition of hydrogen atoms or the removal of oxygen atoms.
On the other hand, oxidation corresponds to a loss of electrons by a molecule, resulting in an increase in its oxidation state. This causes the oxidized molecule to become more positively charged or less negatively charged. In organic reactions, oxidation typically involves the removal of hydrogen atoms or the addition of oxygen atoms.
To recognize the reduced and oxidized organic molecules in a redox reaction, follow these steps:
1. Determine the oxidation state of each atom in the reactants and products.
2. Identify any changes in the oxidation state between the reactants and products.
3. The molecule with a decreased oxidation state has undergone reduction (gained electrons).
4. The molecule with an increased oxidation state has undergone oxidation (lost electrons).
By tracking these changes in oxidation states and charges, you can easily recognize the reduced and oxidized organic molecules in a redox reaction.
For more information on oxidation state see:
https://brainly.com/question/17161178
#SPJ11