Answers
Answer:
Potential energy
Explanation:
The energy accumulated in an entity as a result of its location relative to a neutral level is known as potential energy. If such an object is detected at a height greater than zero, it has gravitational force.
Related Questions
Answer the following about the diagram below:
Label (A), (B) and (C) in the image.
Is the reaction endothermic or exothermic? Explain your answer.
How would adding a catalyst affect the reaction?

Answers
Exothermic reaction
Explanation:Energy diagrams can help us determine how the energy of reactants changes throughout a reaction.
Energy Diagrams
The purpose of energy diagrams is to show how the energy of reactants and products changes over time.
In the diagram, A is the activated complex. This is the intermediate compound that forms from the reactants before the products are made.
B is the activation energy. This is the amount of energy required for the reaction to occur.
C is the energy of reaction. This is the energy that a reaction absorbs or releases.
Energy of Reaction
Exothermic reactions release energy, and endothermic reactions absorb energy. This means that in exothermic reactions the reactants have higher energy than the products. On the other hand, in endothermic reactions, the reactants are lower energy than the product. In this reaction, the reactants are higher energy, so the reaction is exothermic. This means that energy is released, and the energy of reaction will be negative.
Catalyst
A catalyst is a compound that can be added to a reaction to increase the rate of reaction. Catalysts increase the rate of reaction by decreasing the activation energy. This makes the reaction more likely to occur and speeds up the reaction. Catalysts also decrease the energy of the activated complex.
How is wind energy more environmental friendly than heat energy?
Answers
Answer:
Wind is an emissions-free source of energy
Overall, using wind to produce energy has fewer effects on the environment than many other energy sources. Wind turbines do not release emissions that can pollute the air or water (with rare exceptions), and they do not require water for cooling.
Explanation:
so ...this is the question and I hope you get it right one

Answers
Answer:
About 96 grams per mole
Explanation:
The chemical formula of ammonium carbonate is \((NH_4)_2CO_3\). This means that breakdown of moles of atom per mole of molecule is:
2 moles of nitrogen, with a molar mass of around 14
8 moles of hydrogen, with a molar mass of around 1
1 mole of carbon, with a molar mass of about 12
3 moles of oxygen, with a molar mass of about 16
The molar mass of ammonium carbonate is therefore:
2(14)+8(1)+1(12)+3(16)=28+8+12+48=about 96
Hope this helps!
how is using safety equiptment important
Answers
Answer:
You protect yourself and others from numerous injuries. Such safety equipment would be goggles, tongs, gloves(sometimes), hair pulled back, sleeves rolled up, etc.
Explanation:
fill in the blank. "Hydration is a specific example of the phenomenon known generally as __________.
a. solvation
b. disordering
c. dilution
d. salutation
e. condensation"
a. solvation
Answers
Hydration is a specific example of the phenomenon known generally as a. solvation
The act of hydrating involves combining or dissolving an object in water. It is a particular instance of the more general phenomena known as solvation, which is the process by which solvent molecules surround and scatter a solute to create a homogeneous solution. However, hydration explicitly refers to solvation with water as the solvent.
Solvation may also happen with solvents other than water. Solvation is the process through which a solute and solvent interact to stabilise a solute species. Due to its impact on the solubility, reactivity, and behaviour of compounds in solution, solvation is a crucial mechanism in many chemical and biological processes.
Read more about solvation on:
https://brainly.com/question/530845
#SPJ4
how will you increase the solubility of oxygen in water? the partial pressure of oxygen ( p0 , ) is '(\,e9 co 0.21 atm in air at i atm (pext). (a) increase p0 , but keep pext constant (b) decrease p0 , but keep pext constant (c) increase pcxt but keep p0 , constant (d) decrease pext but keep , constant
Answers
By raising the partial pressure of oxygen (p0) above the water's surface, it is possible to improve oxygen's solubility in water.
Henry's Law states that the partial pressure of a gas above a liquid directly correlates with the gas's ability to dissolve in the liquid.Therefore, increasing p0 while maintaining a constant pext is the right answer (a). The partial pressure of oxygen can be increased to do this by raising the oxygen concentration in the air above the water. Other ways to raise p0 can involve raising the water's temperature or lowering the atmospheric pressure over the water's surface.Alternately, lowering p0 (option b) would result in a reduction in the solubility of oxygen in water. Pext growth (option c)By raising the partial pressure of oxygen (p0) above the water's surface, it is possible to improve oxygen's solubility in water. Henry's Law states that the partial pressure of the gas above the liquid directly correlates to how soluble a gas is in a liquid. Other ways to raise p0 can involve raising the water's temperature or lowering the atmospheric pressure over the water's surface.Alternately, lowering p0 (option b) would result in a reduction in the solubility of oxygen in water. The solubility of oxygen in water would not be directly impacted by either option c or option d unless they also changed the partial pressure of oxygen above the water raising
learn more about raising here :
https://brainly.com/question/30128105
#SPJ11
.
PLEASE! I HAVE 20 MINS LEFT :( Two aqueous solutions of AgNO3 and NaCl are mixed. Which of the following diagrams best represents the mixture? For simplicity, water molecules are not shown (Ag + = gray, Cl- = orange, Na + = green, NO ^ - 3 = blue) PLEASE I NEED HELP I ONLY HAVE 15 MINS PLS :'((

Answers
Answer:
diagram C best represents the mixture
ASAPPPP EASYYYY!!!
Total number of single, double, and triple bonds for C2H6(g).
_ _ _
Answers
Answer:
2 single, 0 double, and 1 triple
Explanation:
H-C≡C-H
Carbon disulfide gas and oxygen gas react to form sulfur dioxide gas and carbon dioxide gas. What volume of carbon dioxide would be produced by this reactionif 1.1 L of carbon disulfide were consumed?Also, be sure your answer has a unit symbol, and is rounded to 2 significant digits.08 0 0.20.0X

Answers
Answer: Based on the stoichiometry 1 mole of carbon disulfide will produce 1 mole of carbon dioxide. Similarly we can apply the volume ratio, so 1.1L of carbon disulfide will produce 1.1L of carbon dioxide.
Por qué razón se fomentó la inmigración Europea?
Answers
Hola aquí va la respuesta!
Se fomentó la inmigración europea porque la economía no era buena y vinieron a Paraguay para mejorar su situación.
Hola aquí va la respuesta!
Se fomentó la inmigración europea porque la economía no era buena y vinieron a Paraguay para mejorar su situación.
PLEASE HURRY AND TELL ME THE ANWERS

Answers
Answer:
Explanation:

) determine the weight-average molecular weight of the polymer, and (b) the mean-squared radius of gyration, r2 g in both solvents. (c) is r2 g the same for both polymers? why or why not? (d) if you were to assume this polymer is polystyrene with a kuhn length of b
Answers
By adding up the weights of each chain and dividing by the total number of chains, the average molecular weight can be calculated.
The average molecular weight is a crucial way to describe polymers. The entire weight of the polymer divided by the total number of molecules yields the average molecular weight. chromatography using gel permeation (GPC) Size exclusion chromatography is another name for gel permeation chromatography. It is a common technique for figuring out high molecular weight distribution. Using this method, chemicals are separated based on the size of their molecules. This is also the root-mean-square distance of the molecule's segments from the center of mass for polymer chains. One indicator of the magnitude of the random coil shape that many synthetic polymers adopt in solution or in the amorphous bulk state is the radius of gyration.
To learn more about polymers click here https://brainly.com/question/825578
#SPJ4
if the ka for nh4 =5.6×10−10, find the kb for nh3. assume that the reaction takes place at 25∘c. select the correct answer below: 1.25×10−6 3.49×10−6 1.79×10−5 5.83×10−5
Answers
The Kb for NH3 is 1.79×10−5.
What is the value of Kb for NH3?In aqueous solutions, ammonium hydroxide (NH4OH) partially dissociates to form ammonium (NH4+) and hydroxide (OH-) ions.
The equilibrium constant for this dissociation is known as the Kb value for the reaction NH3 + H2O ⇌ NH4+ + OH-. The Ka and Kb values are related through the autoionization of water (Kw = Ka * Kb).
To find the Kb value for NH3, we can use the given Ka value for NH4+ (5.6×10−10) and the known value of Kw at 25°C (1.0×10−14). By rearranging the equation, Kb = Kw / Ka, we can calculate the Kb value as 1.79×10−5.
Learn more about Kb
brainly.com/question/16816808
#SPJ11
helpppp
a. what are the major aspects of the carbon cycle that carbon cycle? b. what role and impact does increasing methane in the atmosphere have on the temperature of the air?
Answers
It is important to address and mitigate the sources of methane emissions in order to mitigate the impacts of climate change.
A. The major aspects of the carbon cycle include the movement of carbon between the atmosphere, oceans, and land. Carbon is taken up by plants through photosynthesis, and then transferred to animals through the food chain.
When plants and animals die, their bodies decompose, and carbon is released back into the atmosphere or soil. Additionally, human activities such as burning fossil fuels and deforestation have significantly altered the carbon cycle, leading to increased levels of carbon dioxide in the atmosphere.
B. Methane is a potent greenhouse gas, meaning that it traps heat in the Earth's atmosphere and contributes to global warming. When methane is released into the atmosphere, it absorbs infrared radiation and warms the air. As the concentration of methane in the atmosphere increases, so does its impact on the temperature of the air.
This can lead to a range of negative effects, including more extreme weather patterns, rising sea levels, and the loss of biodiversity. Therefore, it is important to address and mitigate the sources of methane emissions in order to mitigate the impacts of climate change.
To know more about photosynthesis, refer here:
https://brainly.com/question/29764662#
#SPJ11
The atomic mass and abundance of Cr-50 is 49.946 amu and 4.3%. The atomic mass and abundance of Cr-52 is 51.941 amu and 83.8%. The atomic mass and abundance of Cr-53 is 52.941 amu and 9.5%. The atomic mass and abundance of Cr-54 is 53.939 amu and 2.4%. Which is the average atomic mass of chromium?
A.51.94 amu
B.52.00 amu
C.52.19 amu
D.53.94 amu
Answers
Answer:
52.00 AMU
Explanation:
(49.946 * 0.043) + (51.941 * 0.838) + (52.941 * 0.095) + (53.939 * 0.024) = 51.998
Make sure to round, 52.00 AMU.
Fluorine is a gas and iodine is a solid, yet all halogens are non-polar and would interact with London dispersion forces. This is a result of
Answers
Answer:
the molecules of each substance attract each other through dispersion (London) intermolecular force.whether a substance is solid, liquid or gas depends on the balance between the kinetic energies of the molecules and their intermolecular attractions.
thank you.
Yo I rly need help plzzz

Answers
the mass of the sun
what is the mass of the sun
Answers
Answer:
1.989 × 10^30 kg
Explanation:
Hope this helps! :3
☁️ Answer ☁️
A solar mass is the mass of the sun. Or, more precisely, it's 1.989 x 10^30 kilograms — about 333,000 Earths. Astronomers use a solar mass as a basic unit of mass.
Dear fool, you will always be a fool.
Happy April Fools Day.
Hope it helps.
Have a nice day noona/hyung
Noble gases are generally
Answers
Answer:
stable
Explanation:
If humans were to inhabit another planet almost just like earth what would we do first before taking off our helmet HINT: For what we do first we have to wait 20 to 30 years for it to _____ big enough to produce a decent amount of ______
Answers

explain 3 different ways that fossils can form?
Answers
Fossils can form in three different ways is Preservation of original remains, Castings and permineralization.
What are fossils and examples?The remnants or evidence of prehistoric life that have been successfully preserved by natural processes are known as fossils. Shells, bones, animal or microbe impressions in stone, exoskeletons, items stored in amber, petrified wood, coal, hair, oil, and Genetic traces are a few examples of fossils.
What makes fossils significant?They provide as a direct link to historical ways of life, ecosystems, and climatic conditions. They show the historical evolution of life, the environment, and the climate, as well as the responses of living organisms to those changes. These lessons are particularly important at this time, as the contemporary world is evolving more and more. Nothing can ever replace a fossil.
To know more about Fossils visit:
https://brainly.com/question/30503344
#SPJ1
One type of neon has 10 neutrons, while other types have 11 neutrons and 12 neutrons. which term best defines the different types of neon atoms in nature?
Answers
One type of neon has 10 neutrons, while other types have 11 neutrons and 12 neutrons. The term best defines the different types of neon atoms in nature is known as Isotopes.
Isotopes are atoms of the similar element that have indifferent numbers of neutrons but the equal number of proton and electrons.
The different in the neutrons results the same element with different masses.
Neon is a chemical element. Its symbol is denoted by Ne.
It is called as Nobel gas and the most stable element.
If talking about the properties it is odorless , uncolored, monoatomic, chemically inactive.
Also called as inert gas.
To know more about Isotopes here :
https://brainly.com/question/11680817?referrer=searchResults
#SPJ4
Answer: isotopes
Explanation:
i took the test
Map of the United notes with latitude and longitude lines. The following cities are labeled: Boise, Denver, Austin, Saint Paul, Madison, Lansing, Indianapolis, Nashville, Atlanta.
What city is located at approximately 45° north latitude and 85° west longitude?
Boise
Lansing
Madison
Nashville
Answers
Based on the information given, there is Lansing city among the options provided that is located at approximately 45° north latitude and 85° west longitude. Option B
To determine the city located at approximately 45° north latitude and 85° west longitude, we can refer to the given list of cities and their corresponding latitude and longitude coordinates.
Based on the latitude and longitude values provided, the city located at approximately 45° north latitude and 85° west longitude is Lansing. Lansing is the capital city of the state of Michigan and is situated in the Lower Peninsula of Michigan.
Boise, Denver, Austin, Saint Paul, Madison, Lansing, Indianapolis, Nashville, and Atlanta are all listed cities, but by examining their approximate latitude and longitude coordinates, we can see that Lansing is the closest match to the given coordinates.
It's important to note that the accuracy of the answer may depend on the precision of the latitude and longitude values provided for each city. However, based on the information given, Lansing is the city that aligns closest to the approximate coordinates of 45° north latitude and 85° west longitude.
Option B
For more such question on longitude visit;
https://brainly.com/question/26115924
#SPJ8
which gas is librated when dilute solution of hydrochloric acid react with metal
Answers
!!!WILL GIVE YOU BRANLIEST!!!!
which option is an example of a physical change??
A breaking chemical bonds
B forming chemical bonds
C nuclear decay
D melting a substance
which option is an example of a chemical change
A ironing a shirt
B melting cheese
C grinding powder
D fireworks exploding
Answers
Answer:
D
Explanation:
Melting of ice is a physical change as there no new compound is forming only state is changing So Option D is correct option. For second question Option D is correct option as combustion reaction is taking place.
What is chemical change?Chemical change is a reaction in which breaking and forming of new bonds take place. One molecule completely converted to totally different molecule. The number of atoms remains same
One famous example of chemical change is the rusting of iron. The color, odor and arrangements of atoms in products are very much different from reactant. Chemical change are mostly irreversible in nature. Physical change is just opposite of chemical change. Phase change is an example of physical change.
Thus option D is correct option in both cases.
Learn more about the chemical change, here:
https://brainly.com/question/16279122
#SPJ2
PLS HELP ASAP!
How many years?
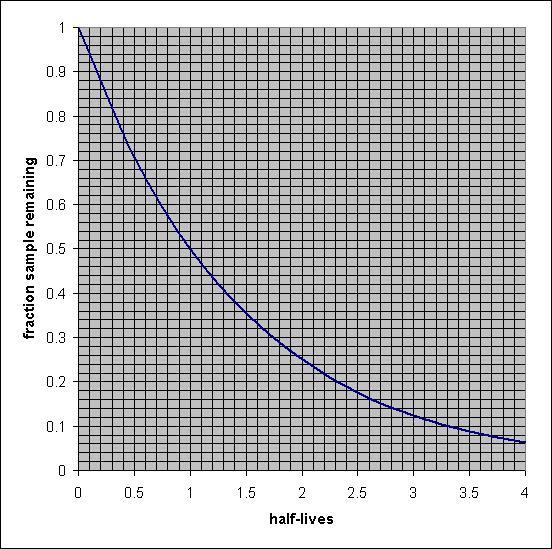
The radioactive isotope carbon-14 is used for radiocarbon dating. The half-life of carbon-14 is 5.73×103 years.
A wooden artifact in a museum has a 14C to 12C ratio that is 0.745 times that found in living organisms. Estimate the age of the artifact.
use graph
Answers
Answer:
about 5 years i think
Explanation:
I've tried all the methods i know
it's about 5 years
hope it helps
The age of the artifact is equal to 2.5 × 10³ years when the half-life of carbon- 14 is 5.73 × 10³ years.
What is the half-life period?The half-life of a radioactive element is defined as the time that is required to decrease the original quantity of a radioactive isotope to half after decay.
The half-life of a radioactive isotope is the feature of the element and does not depend upon the actual amount of the radioactive isotope.
Given, the ratio of the initial and left amount of ¹⁴C is :
N = 0.745 N₀
The half-life period of the ¹⁴C isotope = 5.73 × 10³ years
The rate constant of the decay of radioisotope can be calculated from the below-mentioned formula:
\(\displaystyle k=\frac{0.693}{t_{1/2}}\)
\(\displaystyle k=\frac{0.693}{5.73 \times 10^3}\)
k = 0.123 × 10⁻³ yr⁻¹
The age of the artifact can be calculated as:
\(\displaystyle t =\frac{2.303}{k} log\frac{N_0}{N}\)
\(\displaystyle t =\frac{2.303}{0.123\times 10^{-3}} log\frac{N_0}{0.735 N_0}\)
t = 2.5 × 10³ years
Learn more about the half-life period, here:
brainly.com/question/14521252
#SPJ2
Element X has an electronegativity of 2. 5 and Y has an electronegativity of 9. X is a ____ and Y is a _____. A) Metal, nonmetal
B) Nonpolar Metal, Metal C) nonmetal, Metal
D) Metal, nonpolar metal
Answers
Element X has an electronegativity of 2. 5 and Y has an electronegativity of 9. X is a metal and Y is a non-metal.
The correct answer is option a.
Element X has a lower electronegativity than Element Y, which means that Element X is a metal and Element Y is a nonmetal.
The capacity of an atom to draw electrons towards itself when it establishes a chemical connection with another atom is measured as electronegativity. It is a fundamental feature of atoms and is commonly measured on the Pauling scale, which provides numerical values ranging from 0.7 (for the least electronegative element, cesium) to 4.0 (for the most electronegative element, uranium) (for the most electronegative element, fluorine).
For more such questions on electronegativity, click on:
https://brainly.com/question/10531792
#SPJ11
Match each part of the atom with its identity from the list below.

Answers
Answer:
Nucleus: Choice C
Electron: Choice E
Proton: Choice A
Neutron: Choice B
Energy Level: Choice D
Explanation:
1. Nucleus contains the protons and neutrons.
2. Electrons surround the nucleus and have a negative charge.
3. Protons are positively charged and found in the nucleus.
4. Neutrons have a neutral charge and are found in the nucleus.
5. The energy level refers to the electron orbital.
1. 3 Cu + 8HNO3 → 3 Cu(NO3)2 + 2 NO + 4 H2O
In the above equation, how many grams of water can be made when 2.1 moles of HNO3 are consumed?
Round your answer to the nearest tenth. If you answer is a whole number like 4, report the answer as 4.0
Use the following molar masses. If you do not use these masses, the computer will mark your answer incorrect.:
Element Molar Mass
Hydrogen 1
Nitrogen 14
Copper 63.5
Oxygen 16
2. S + 6 HNO3 → H2SO4 + 6 NO2 + 2 H2O
In the above equation, how many grams of water can be made when 14.1 moles of HNO3 are consumed?
Round your answer to the nearest tenth. If you answer is a whole number like 4, report the answer as 4.0
Use the following molar masses. If you do not use these masses, the computer will mark your answer incorrect.:
Element Molar Mass
Hydrogen 1
Nitrogen 14
Sulfur 32
Oxygen 16
3. 2 NH3 + 3 CuO → 3 Cu + N2 + 3 H2O
In the above equation, how many grams of N2 can be made when 16.7 moles of CuO are consumed?
Round your answer to the nearest tenth. If you answer is a whole number like 4, report the answer as 4.0
Use the following molar masses. If you do not use these masses, the computer will mark your answer incorrect.:
Element Molar Mass
Hydrogen 1
Nitrogen 14
Copper 63.5
Oxygen 16
4. 2 NH3 + 3 CuO --> 3 Cu + N2 + 3 H2O
In the above equation how many moles of N2 can be made when 160.9 grams of CuO are consumed?
Round your answer to the nearest tenth. If you answer is a whole number like 4, report the answer as 4.0
Use the following molar masses. If you do not use these masses, the computer will mark your answer incorrect.:
Element
Molar Mass
Hydrogen
1
Nitrogen
14
Copper
63.5
Oxygen
16
Answers
1. To solve for the grams of water produced, we need to use stoichiometry. First, we need to convert 2.1 moles of HNO3 to moles of water. From the balanced equation, we can see that for every 8 moles of HNO3, 4 moles of water are produced. Therefore, for 2.1 moles of HNO3:
2.1 moles HNO3 x (4 moles H2O/8 moles HNO3) = 1.05 moles H2ONext, we can use the molar mass of water to convert moles to grams:1.05 moles H2O x 18 g/mol = 18.9 gRounded to the nearest tenth, the answer is 18.9 grams of water.
2. Similarly, we need to use stoichiometry to find the grams of water produced. For 14.1 moles of HNO3:
14.1 moles HNO3 x (2 moles H2O/6 moles HNO3) = 4.7 moles H2OConverting moles to grams using the molar mass of water:4.7 moles H2O x 18 g/mol = 84.6 gRounded to the nearest tenth, the answer is 84.6 grams of water.
3. To find the grams of N2 produced, we need to first convert 16.7 moles of CuO to moles of N2. From the balanced equation, we can see that for every 3 moles of CuO, 1 mole of N2 is produced. Therefore, for 16.7 moles of CuO:
16.7 moles CuO x (1 mole N2/3 moles CuO) = 5.56 moles N2Next, we can use the molar mass of N2 to convert moles to grams:5.56 moles N2 x 28 g/mol = 155.7 gRounded to the nearest tenth, the answer is 155.7 grams of N2.
4. To find the moles of N2 produced, we need to first convert 160.9 grams of CuO to moles. From the molar mass of CuO, we can see that 1 mole of CuO weighs 79.5 g.
160.9 g CuO x (1 mole CuO/79.5 g) = 2.02 moles CuOFrom the balanced equation, we can see that for every 3 moles of CuO, 1 mole of N2 is produced. Therefore, for 2.02 moles of CuO:2.02 moles CuO x (1 mole N2/3 moles CuO) = 0.673 moles N2Rounded to the nearest tenth, the answer is 0.7 moles of N2.Answers:1. 18.9 grams of water2. 84.6 grams of water3. 155.7 grams of N24. 0.7 moles of N2
Does the variable increase(1) or decrease(1) under the described conditions?
1. What happens to pressure as volume
increases?
2. What happens to pressure as temperature decreases?
3. What happens to volume as temperature decreases?
4. What happens to volume as the number of moles increase?

Answers
Answer:
Explanation:
Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. In fact, if the volume increases by a certain factor, the pressure decreases by the same factor, and vice versa. Volume-pressure data for an air sample at room temperature are graphed in Figure 5.
Because the volume has decreased, the particles will collide more frequently with the walls of the container. ... When the volume decreases, the pressure increases. This shows that the pressure of a gas is inversely proportional to its volume. This is shown by the following equation - which is often called Boyle's law.
The kinetic energy of the gas molecules increases, so collisions with the walls of the container are now more forceful than they were before. As a result, the pressure of the gas doubles. Decreasing the temperature would have the opposite effect, and the pressure of an enclosed gas would decrease.
For a fixed mass of gas at constant temperature, the volume is inversely proportional to the pressure. That means that, for example, if you double the pressure, you will halve the volume. If you increase the pressure 10 times, the volume will decrease 10 times.
Temperature, pressure, volume and the amount of a gas influence its pressure.
Gay Lussac's Law - states that the pressure of a given amount of gas held at constant volume is directly proportional to the Kelvin temperature. If you heat a gas you give the molecules more energy so they move faster. This means more impacts on the walls of the container and an increase in the pressure.
i really hope some of this helped i would put more but its a lot too type
