Part A
Determine the value of Kp for the following reaction:
4HCl(g)+O2(g)⇌2Cl2(g)+2H2O(g)
Express the equilibrium constant to three significant digits.
Part B
Determine the value of Kp for the following reaction:
Cl2(g)+H2O(g)⇌2HCl(g)+1/2O2(g)
Express the equilibrium constant to three significant digits.
Part C
What is the value of Kc for the reaction in Part B?
Express the equilibrium constant to three significant digits.
Answers
The value of Kp = 13.2 for first and Kp for second is 0.274. The value for Kc = 0.0344
Part A :
4HCl(g)+O2(g) <-----------------> 2Cl2(g)+2H2O(g)
Kp = Equilibrium constant calculated from the partial pressures.
Kp = [Cl2]^2 [H2O]^2 / [HCl]^4 [O2]
= (1/Kp1)
= 1 / 7.55×10−2
= 13.2
Kp = 13.2
Part B :
Cl2(g) + H2O(g) <-----------------> 2HCl(g) + 1/2O2(g)
Kp = [HCl]^2 [O2]^(1/2) /[Cl2] [H2O]
= sqrt (Kp1)
= sqrt (7.55×10−2)
= 0.274
Kp = 0.274
Part C:
Dn = 5/2 - 2 = 1/2 = 0.5
Kp = Kc (RT)Dn
Kc = Equilibrium constant measured in moles per liter.
0.274 = Kc (0.0821 x 773)^0.5
Kc = 0.0344
To learn more about equilibrium click here:
https://brainly.com/question/5537989
#SPJ4
Related Questions
Write the balanced chemical equation for the reaction
of solid carbon with oxygen gas to form carbon monoxide gas.
Answers
Answer:
2C +O2 - COExplanation:
mark me brainliest
The balanced chemical equation for the reaction of solid carbon with oxygen gas to form carbon monoxide gas is 2 C + O₂\(\rightarrow\) 2 CO.
What is chemical equation?Chemical equation is a symbolic representation of a chemical reaction which is written in the form of symbols and chemical formulas.The reactants are present on the left hand side while the products are present on the right hand side.
A plus sign is present between reactants and products if they are more than one in any case and an arrow is present pointing towards the product side which indicates the direction of the reaction .There are coefficients present next to the chemical symbols and formulas .
The first chemical equation was put forth by Jean Beguin in 1615.By making use of chemical equations the direction of reaction ,state of reactants and products can be stated. In the chemical equations even the temperature to be maintained and catalyst can be mentioned.
Learn more about chemical equation,here:
https://brainly.com/question/28294176
#SPJ2
A single atom of an element has 21 neutrons, 20 electrons, and 20 protons, which elementis it?
Answers
Answer:
Calcium
Explanation:
The number of protons is the atomic number of the element. Number 20 on the periodic table is Calcium.
Easyyyyy plz ...................
Permanent hardness of water is caused by dissolved :-
A) CaCl
B) Ca(HCO3)2
C) Mg(HCO3)2
D) NaOH
Answers
Answer:
The answer is B
Explanation:
Answer:
Hello!
Your answer is B) Ca(HCO3)2!!
Explanation:
Hope this helps you!!
itsMATT04
:>
Which feature forms at Earth's surface from the cooling of lava?
O extrusion
fault
O intrusion
unconformity
Answers
Extrusion is the feature form at Earth's surface from the cooling of lava.
What are extrusive igneous rocks?They are the ones that originate from a sudden cooling of the incandescent magma when it comes to the surface, which means that there is no time for crystals to form, either partially or totally.
Characteristics of extrusive igneous rocksThese are rocks formed mainly by silicate minerals.Pyroclastics are the product of explosive volcanic eruptions and contain rock fragments of different origins, they can be of many shapes and sizes.Therefore, we can conclude that typical volcanic rocks are formed by the rapid cooling of lava and pyroclastic fragments.
Learn more about extrusive igneous rocks here: https://brainly.com/question/1340933
Answer:
A.
Explanation:
The formation of protons over neutrons (thus producing about a 7:1 ratio) was favored in the early universe because:____.
a. it takes more energy to create a proton from a neutron than a neutron from a proton. b. it takes more energy to create a neutron from a proton than a proton from a neutron. c. the more massive neutrons made better targets for nucleon collisions. d. the positively charged protons were repelled away from the explosion.
Answers
Because it requires more energy to create a neutron from a proton than it does to create a proton from a neutron, protons were formed more frequently than neutrons in the early universe. The correct answer is option b.
To find the answer, we need to know more about the early universe.
How the formation of proton over neutrons was favored in the early universe?A neutron is produced with greater energy than a proton. However, later on, some of the protons were changed into neutrons. Contrary to some claims, the proton is a stable particle that never decays, but the neutron is unstable outside of the nucleus and decays with a half-life of around 10.5 minutes. However, very few would have had time to decay on the timeline you mention in your question. Every matter particle should have been accompanied by an antimatter particle, and every proton, neutron, and electron, by an anti-neutron and a positron, respectively. Where did all the antimatter go is the great mystery. There have been a few attempts to explain this, but they have failed.Thus, we can conclude that, the correct answer is option b.
Learn more about the early universe here:
https://brainly.com/question/13191712
#SPJ1
A series of solutions is used to plot a standard curve that is then used to find parameters about an unknown. In the experiments conducted in this course, we plotted absorbance vs. concentration. In a General Chemistry lab (not limited to Chemistry 105), which of the following is not a typical calibration plot? Select one: a.Precipitating mass vs amount of titrant added b.Intensity of color vs concentration c.Concentration vs volume of vessel d.Electrical conductivity vs concentration
Answers
In a General Chemistry lab, the calibration plot that is not typical is "electrical conductivity vs concentration".
So, the correct answer is D.
A calibration plot is a graph of a known quantity that is used to determine an unknown quantity.
In this case, a series of solutions is used to plot a standard curve that can then be used to find parameters about an unknown. In the experiments conducted in this course, absorbance vs concentration was plotted, which is a common calibration plot used in chemistry.
Other examples of calibration plots include precipitating mass vs amount of titrant added, intensity of color vs concentration, and concentration vs volume of vessel.
Electrical conductivity vs concentration is not typically used because it is not a reliable method for determining concentration.
Hence, the answer of the question is D.
Learn more about concentration at https://brainly.com/question/29729322
#SPJ11
We are studying the ideal gas law. In this discussion, you will be trying your hand at applying one of the ideal gas laws to a real world situation. Consider a situation that involves an ideal gas law and discuss how you would apply your chosen ideal gas law to the situation. Generate an ideal gas law question based on this situation.
Please do not forget to generate a question.
Answers
The ideal gas law, which relates the pressure, volume, temperature, and number of moles of an ideal gas, can be applied to real-world situations. By considering a specific scenario and applying the ideal gas law, we can analyze the behavior of gases and make predictions about their properties.
Let's consider a situation where a scuba diver is exploring underwater at a depth of 30 meters. We can apply the ideal gas law, specifically the form known as Boyle's law, which states that the pressure and volume of a gas are inversely proportional at constant temperature.
Question: How does the pressure of the gas in the scuba tank change as the diver descends to a depth of 30 meters, assuming the temperature remains constant?
To answer this question, we can use the ideal gas law equation PV = nRT, where P is the pressure, V is the volume, n is the number of moles of gas, R is the ideal gas constant, and T is the temperature. By keeping the temperature constant, we can observe the relationship between pressure and volume as the diver descends and calculate the change in pressure based on the change in volume.
To learn more about Boyle's law click here:
brainly.com/question/30367133
#SPJ11
pls help meeee 06.01 BIOTECHNOLOGY
Biotechnology Activity
Now that you are more familiar with the benefits, risks, and impacts of biotechnology, it is time for you to take a stand! Your task is to choose one of the following types of biotechnology: genetic engineering, cloning, or artificial selection.
You will then write a one- to two-paragraph summary describing your chosen type of biotechnology. You will then need to argue for either the benefits or the risks of your chosen type. Your arguments should present your position, and then give the evidence that led you to this position. Be sure to include the following in your argument:
a description of your chosen type of biotechnology (genetic engineering, cloning, or artificial section)
one benefit or one risk for the individual (based on whether you are for or against it)
one benefit or one risk for society (based on whether you are for or against it)
one benefit or one risk for the environment (based on whether you are for or against it)
A picture (you may hand draw, take photos in nature, or use stock images)
You may get creative on this activity. You may choose to create a brochure, write a letter, or create a presentation using software. If you are unsure if your idea or software for a presentation will work, contact your instructor for assistance.
Answers
A type of biotechnology called artificial selection is deliberately breeding plants or animals to promote or eliminate specific desirable qualities. Crops may be carefully bred by farmers, for instance, to increase their resistance to pests or drought.
Artificial selection is a type of biotechnology that involves selectively breeding plants or animals to enhance or suppress certain desirable traits.
For example, farmers may selectively breed crops to make them resistant to pests or droughts.
One benefit of artificial selection for individuals is that it can lead to higher crop yields, which can result in increased profits for farmers. However, one risk of artificial selection for individuals is that it can lead to the loss of genetic diversity in crops, which can make them more vulnerable to disease outbreaks.
One benefit for society is that artificial selection can help to alleviate hunger and food shortages by producing more food. However, one risk for society is that artificial selection can lead to the creation of monocultures, which are vulnerable to pests and disease outbreaks.
One benefit for the environment is that artificial selection can lead to the production of crops that require fewer pesticides and herbicides, which can reduce pollution. However, one risk for the environment is that artificial selection can lead to the loss of biodiversity, which can negatively impact ecosystems.
Attached is an image of a farmer selectively breeding crops.
For more such questions on biotechnology, click on:
https://brainly.com/question/29772046
#SPJ11

23. A certain sample of nitrogen gas consists of 9.26 1022 nitrogen atoms.
(a) How many moles of N atoms are present in this sample ?
(b) If the gas is entirely in molecular form, how many molecules are present in this
sample?
(c) What is the mass of the sample in gramme?
Answers
Answer:
a) 0.154 moles of N2
b) 9.27 × 10^22 molecules
c) 4.312 g
Explanation:
a)
1 mole of N2 contains 6.02 × 10^23 atoms
x moles of N2 contains 9.26 × 10^22 atoms
x= 9.26 ×10^22/6.02 × 10^23
x= 1.54 ×10^-1
x= 0.154 moles of N2
b)
1 mole of N2 contains 6.02 × 10^23 molecules
0.154 moles of N2 contains 0.154 moles × 6.02 × 10^23 = 9.27 × 10^22 molecules
c)
Molar mass of the nitrogen gas= 28 gmol^-1
Number of moles of nitrogen gas = 0.154 moles
Number of moles = mass/molar mass
0.154 moles= mass/28 gmol^-1
Mass= 0.154 moles × 28gmol^-1
Mass= 4.312 g
A solution of NaCl was prepared in the following manner: 0.0842 g of NaCl is massed out on an analytical balance. The solid is transferred to a 25.00 mL volumetric flask. Deionized water is added to the flask such that the bottom of the meniscus is at the line. A 1.00 mL aliquot of the stock solution is transferred to a 50.00 mL volumetric flask using a volumetric pipet and diluted to volume. 6. Calculate the concentration of NaCl in the resulting solution in mg/L NaCl. (answer = 67.4 mg/L) 7. Calculate the concentration of NaCl in the resulting solution using propagation of error through the calculation. Use the manufacturer's tolerance values as the absolute error. The tolerances can be found in Chapter 2 of the Harris text. Assume a Class 1 balance and Class A glassware. Treat the tolerances as random error. (answer = 67.4+0.4 mg/L) 8. Identify 2 possible sources of random (indeterminate) error. Identify 2 possible sourses of systematic (determinate) error.
Answers
Two possible sources of systematic (determinate) error in the experiment are; Incorrect calibration of volumetric glasswareIncorrect mass of NaCl
To calculate the concentration of NaCl in the resulting solution in mg/L NaCl, we can use the formula; Concentration (mg/L) = (Mass of solute ÷ Volume of solution in L) × 1000 g / 1 mg NaCl is present in the stock solution of 25 mL. So, the mass of NaCl in the solution would be;0.0842 g ÷ 25 mL = 0.00337 g/mL. Now, in the resulting solution, a 1.00 mL aliquot of the stock solution is transferred to a 50.00 mL volumetric flask and diluted to volume. Therefore, the volume of the resulting solution is 50.00 mL. We will substitute these values in the formula, Concentration (mg/L) = (0.00337 g/mL ÷ 50 mL) × 1000 g / 1 mg concentration (mg/L) = 67.4 mg/L. Therefore, the concentration of NaCl in the resulting solution in mg/L NaCl is 67.4 mg/L.7. Concentration = 67.4 mg/LTolerance = 4.28 mg/LTotal concentration = 67.4 + 4.28 mg/L = 71.68 mg/LWe round off this value to one decimal place; Total concentration = 71.7 mg/LTherefore, the concentration of NaCl in the resulting solution using propagation of error through the calculation is 67.4+0.4 mg/L.8. Two possible sources of random (indeterminate) error in the experiment are; Errors in temperature measurement. Errors in measurement of water volume. Two possible sources of systematic (determinate) error in the experiment are; Incorrect calibration of volumetric glasswareIncorrect mass of NaCl.
Learn more about NaCl
https://brainly.com/question/32275922?
#SPJ11
if there is 16.66 g p4 and excess cl2 present, the reaction yields 54.8 g pcl3. calculate the percent yield for the reaction.
Answers
The percent yield of a reaction is a measure of how efficiently the reaction proceeds, calculated by comparing the actual yield to the theoretical yield. In this case, the reaction involves 16.66 g of phosphorus (P4) and excess chlorine (Cl2), resulting in the production of 54.8 g of phosphorus trichloride (PCl3). To calculate the percent yield, we need to determine the theoretical yield first. The percent yield for the reaction is approximately 74.3%.
The molar mass of P4 is 123.88 g/mol, while the molar mass of PCl3 is 137.33 g/mol. Based on the balanced chemical equation, 1 mol of P4 reacts with 6 mol of Cl2 to produce 4 mol of PCl3. Therefore, the molar ratio between P4 and PCl3 is 1:4.
To calculate the theoretical yield, we convert the given mass of P4 into moles using its molar mass:
16.66 g P4 * (1 mol P4 / 123.88 g P4) = 0.1343 mol P4
Using the molar ratio, we can determine the moles of PCl3 that should be produced:
0.1343 mol P4 * (4 mol PCl3 / 1 mol P4) = 0.5372 mol PCl3
Finally, we convert the moles of PCl3 into grams using its molar mass:
0.5372 mol PCl3 * (137.33 g PCl3 / 1 mol PCl3) = 73.84 g PCl3
The theoretical yield of PCl3 is calculated to be 73.84 g. To determine the percent yield, we divide the actual yield (54.8 g) by the theoretical yield (73.84 g) and multiply by 100:
Percent Yield = (54.8 g / 73.84 g) * 100 = 74.3%
Therefore, the percent yield for the reaction is approximately 74.3%. This value indicates that the reaction produced 74.3% of the expected amount of PCl3 based on the given amount of P4. The lower percent yield suggests that there may have been some inefficiencies or losses during the reaction, resulting in a reduced yield of the desired product.
Learn more about theoretical yield here:
brainly.com/question/33781695
#SPJ11
can someone help me solve the questions below using the data table below please
DATA TABLE:
Mass of flask and vinegar solution- 25.17g
Mass of flask- 15.12g
Volume of vinegar solution (in mL)- 10.00ml
Initial volume of NaOH (in mL)-0.00ml
Final volume of NaOH (in mL)-39.00ml
CALCULATIONS:
Mass of vinegar solution- 10.0503g
Volume of NaOH used in titration (in mL)-39.00ml

Answers
The percent by mass of acetic acid in the vinegar is 2.33%.
Below are the steps to solve the given problem using the data table given below:
Step 1: Calculate the mass of the vinegar. Given,Mass of flask and vinegar solution- 25.17gMass of flask- 15.12gMass of vinegar solution = Mass of flask and vinegar solution - Mass of flask= 25.17 g - 15.12 g= 10.05 g
Step 2: Calculate the moles of NaOH used in the titration.Molarity of NaOH solution is 0.1 M.Moles of NaOH = Molarity × Volume of NaOH usedMoles of NaOH = 0.1 M × 39.00 mL (since the initial volume of NaOH is 0.00 mL)Moles of NaOH = 0.0039 moles
Step 3: Determine the moles of acetic acid used in the reaction.The balanced chemical equation for the reaction between NaOH and acetic acid (the main component of vinegar) is given below:CH3COOH + NaOH → CH3COONa + H2OMoles of NaOH = Moles of CH3COOH (since they react in a 1:1 ratio)Moles of CH3COOH = 0.0039 moles
Step 4: Calculate the mass of acetic acid used in the reaction.Molar mass of acetic acid is 60.05 g/mol.Mass of CH3COOH = Moles of CH3COOH × Molar mass of CH3COOH= 0.0039 moles × 60.05 g/mol= 0.234 gStep 5: Calculate the percent by mass of acetic acid in the vinegar.Percent by mass of acetic acid = (Mass of CH3COOH / Mass of vinegar solution) × 100%= (0.234 g / 10.05 g) × 100%= 2.33%.
for such more questions on mass
https://brainly.com/question/24191825
#SPJ8
What Is the energy of a photon with a wavelength of 21nm? I
Answers
The energy of a photon with a wavelength of 21 nm can be calculated using the equation E = hc/λ, where E represents the energy of the photon, h is Planck's constant (6.626 x 10^-34 J·s), c is the speed of light (3.00 x 10^8 m/s), and λ is the wavelength in meters.
To calculate the energy of the photon with a wavelength of 21 nm, we first need to convert the wavelength from nanometers to meters. There are 1 billion nanometers in a meter, so 21 nm is equal to 21 x 10^-9 meters.
Substituting the values into the equation, we have:
E = (6.626 x 10^-34 J·s) * (3.00 x 10^8 m/s) / (21 x 10^-9 m)
By performing the calculation, we find that the energy of a photon with a wavelength of 21 nm is approximately 9.971 x 10^-17 Joules.
To learn more about Wavelength - brainly.com/question/31143857
#SPJ11
What is used to determine the number of each atom in an ionic formula
Answers
Answer:
The charge carried by each ion (oxidation state of each atom)
Explanation:
If we have an ionic compound and we want to write its formula, we must first know the magnitude of charge on each ion (shown as oxidation state of the atoms involved) because the magnitude of charge on each ion is eventually crisscrossed and gives the subscript (number of atoms) for each atom in the formula.
For instance, let us write the formula of calcium bromide. Ca has a charge of +2 while Br has a charge of -1. If we exchange the charges and ignore the signs such that the crisscrossed charges form subscripts we can now write; \(CaBr_{2}\).
What is a "siege machine"?
Answers
Answer:
A siege engine is a device that is designed to break or circumvent heavy castle doors, thick city walls and other fortifications in siege warfare. Some are immobile, constructed in place to attack enemy fortifications from a distance, while others have wheels to enable advancing up to the enemy fortification.
Explanation:
Please help. The moon is Earth's only natural satellite. The following are characteristics of Earth's moon, except ...
A)
Temperature ranges from 130 °C in direct sunlight to -180 °C at night,
because there is no atmosphere.
There are traces of water on the surface that may have originated deep
underground
B
C)
The moon's surface is composed of craters, maria and highlands,
D
The moon s about 1/2 the size of Earth s diameter
Answers
Answer:
d) The moon s about 1/2 the size of Earth s diameter
Explanation:
Why are there more sodium ions in the sulfide compound
Answers
Answer:
2 Na
Explanation:
2 Na atoms per 1 Sulfide atom
hope this helps
I have 345 ml of a 1. 5 m nacl solution. If i boil the water until the volume of the solution is 250 ml, what will the molarity of the solution be?.
Answers
After boiling the water, the molarity of the NaCl solution will be approximately 2.07 M.
To find the new molarity of the solution, we need to use the equation M1V1 = M2V2, where M1 is the initial molarity, V1 is the initial volume, M2 is the final molarity, and V2 is the final volume.
Given that the initial volume is 345 ml and the final volume is 250 ml, we can find V1 as follows:
M1V1 = M2V2
1.5 M x 345 ml = M2 x 250 ml
M2 = (1.5 M x 345 ml) / 250 ml
M2 = 2.07 M
Therefore, the molarity of the solution after boiling will be 2.07 M. This means that the concentration of NaCl in the solution has increased due to the removal of water through boiling.
Learn more about molarity here:
https://brainly.com/question/8732513
#SPJ11
Beryllium has 2 valence
electrons. Which element could
pair with it in a 1 Be : 1 atom
ratio?
A. Li (1 valence electron)
B. P (5 valence electrons)
C. Br (7 valence electrons)
D. Se (6 valence electrons)
Answers
Beryllium with 2 valence electrons would be able to pair with Se with 6 valence electrons. Option D.
Formation of bondsAtoms form bonds with one another based on the number of valence electrons present in their outermost shell. Electrons are either donated, received, or paired.
In order for bonds to form, the participating atoms try to assume an octet structure, In other words, each atom tries to have a completely filled outermost shell. Thus, atoms with only a few electrons in their outermost shells readily donate them while those with just a few electrons remaining to fill up their outermost shell readily accept them.
Be has 2 outermost electrons, Se has 6. Be will readily donate the 2 outermost electrons while Se will readily accept 2 electrons for completely filled shell.
More on formation of bonds can be found here: https://brainly.com/question/11539094
#SPJ1
Type your answer in the space below, rounded to the nearest whole number.
Answers
Answer:
Follow my insatagram onlypiccolo for answer
Explanation:
thanks!
Which reaction sequence best accomplishes the given transformation? ? Br OH NaOEt 1) Hg(OAC)2, H2O 2) NaBHA о t-BuOK 1) BHz - THE 2) H2O2, NaOH t-BuOK 1) Hg(OAc)2, H2O 2) NaBHA O NaOET 1) BH, THE 2) H2O2, NaOH
Answers
The best reaction sequence for the given transformation is BrOH → NaOEt → BHz-THE → H2O2, NaOH.
The given transformation involves converting BrOH to BHz-THE. The first step involves the substitution of the hydroxyl group with a sodium ethoxide ion, resulting in the formation of an ether linkage. This is accomplished using NaOEt as the reagent.
In the second step, the ether linkage is cleaved using borane-THF complex (BHz-THE) to yield an alkene. This reaction is regioselective, and the boron atom in the borane complex attacks the less hindered carbon atom of the ether linkage to form an intermediate that subsequently undergoes hydrolysis to yield the alkene.
Finally, the alkene is converted to the desired product using hydrogen peroxide and sodium hydroxide. This reaction is an oxidative cleavage reaction that cleaves the alkene at the double bond to yield two carbonyl compounds. The reaction is carried out under basic conditions, and the resulting products are stabilized by the formation of carboxylate ions.
Learn more about hydrolysis here: https://brainly.com/question/10840252
#SPJ11
Classify each chemical reaction:Reaction2H₂O₂(1)→ 2H₂O(1) + 0₂ (8)K₂SO4 (aq) + Ba(NO3)₂(aq) → 2KNO3(aq) + BaSO4(s)PbCl₂ (aq) + FeSO4 (aq) → FeCl₂ (aq) + PbSO4(s)Typechoose onechoose one✓ choose onecombinationdecompositionsingle substitutionmetathesisnone of the aboveO
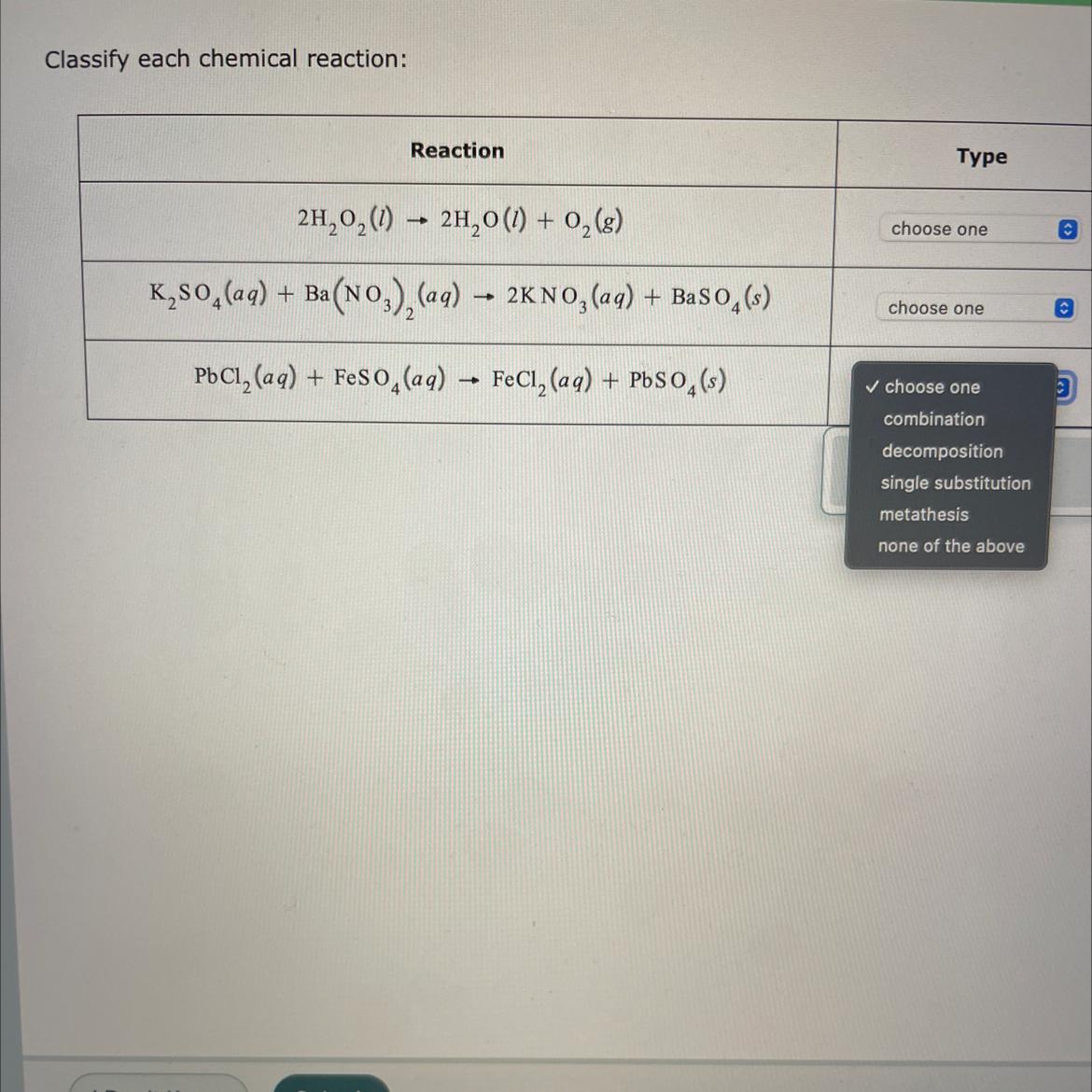
Answers
A decomposition reaction is a chemical reaction in which one reactant breaks down into two or more products.
A metathesis reaction is a chemical reaction in which the positive ions and negative ions present in the reactants appear to exchange partners.
Find the standard molar enthalpy for the reaction C(s) + ½ O2(g) → CO(g)
Answers
The standard molar enthalpy for the reaction C(s) + ½ O2(g) → CO(g) is -111KJ.
What exactly are molar enthalpy and enthalpy?Molar enthalpy is the amount of energy per mole. In light of this, the primary distinction between enthalpy and molar enthalpy is that the former refers to the total heat content of a thermodynamic system, whereas the latter refers to the total heat per mole of reactant in the system.
C(s) + O₂(g) → CO₂(g) ΔHO = -394 kJ ----(1)
CO₂(g) → CO(g) + 1/2O₂(g) ΔHO = +283 kJ -----(2)
Adding 1 & 2
C(s) + ½ O₂(g) → CO(g)
ΔHO = -394 kJ + 283 kJ
ΔHO = -111KJ.
To know more about molar enthalpy visit:
https://brainly.com/question/3207013
#SPJ1
Complete question is " Find the standard molar enthalpy for the reaction C(s) + ½ 02(g) → CO(g)
Given that
C(s) + O2(g) → CO2(g) AHO = -394 kJ
CO2(g) → CO(g) + ¹/2O2(g) AHO = +283 kJ ".
3. Describe what happens to chemical bonds as a chemical reaction is taking place.
4. Give 3 examples on a daily basis of chemical reactions.
5. What is the difference between a chemical formula and a chemical equation?
Answers
Answer:
1. In a chemical reaction, the atoms and molecules that interact with each other are called reactants. ... No new atoms are created, and no atoms are destroyed. In a chemical reaction, reactants contact each other, bonds between atoms in the reactants are broken, and atoms rearrange and form new bonds to make the products.
2. Given below are some of the examples of the chemical reactions in our daily life:
Photosynthesis.
Combustion.
Aerobic cellular respiration.
Anaerobic respiration including the process fermentation.
Metathesis reactions, for example, vinegar and baking soda.
Oxidation which includes rusting.
Digestion.
3. A chemical formula is the combination of atomic symbols that designate a particular chemical compound, or a substance with two or more different elements. ... A chemical equation shows one or more chemical compounds (reactants) being converted into different chemical species called products.
Practice problem 9.4
the equilibrium constant for the system at this temperature?
6.4 x 10 mol/dm³ of N2, 1.7 x 10 mol/dm³ of O2 and 1.1 *10^ - mol/dm³ of NO. What is
An equilibrium mixture of N_{2} U_{2} and NO gases at 1500K is determined to consist of
Answers
The equilibrium constant for the system at the given temperature is 1.1×10⁻⁵
What is equilibrium constant?Equilibrium constant for a given is defined as the concentration of the products raised to their coefficient to the concentration of the reactants raised to their coefficient
For example:
eB <=> cD
The equilibrium constant for the reaction above is given as
Ke = [D]^c / [B]^e
How to determine the equilibrium constant N₂(g) + O₂(g) <=> 2NO(g)[N₂] = 6.4×10⁻³ M[O₂] = 1.7×10⁻³ M[NO] = 1.1×10⁻⁵ MEquilibrium constant (Ke) =?The equilibrium constant for the reaction reaction can be obtained as follow
Ke = [NO]² / [N₂][O₂]
Ke = [1.1×10⁻⁵]² / [6.4×10⁻³][1.7×10⁻³]
Ke = 1.1×10⁻⁵
Thus, the equilibrium constant for the reaction at the given temperature is 1.1×10⁻⁵
Learn more about equilibrium constant:
https://brainly.com/question/17960050
#SPJ1
Complete question
See attached photo

How many moles of Li3PO4 are in 2.2 L of a 0.6 M solution of Li3PO4? (remember Molarity=moles of solute/liters of solution)
Answers
Answer:
5 is the answer since I did this and got it rigt
If 2 grams of water absorbs 20 calories of heat (assume not during a state change), the resulting water temperature change is?
Answers
If 2 grams of water absorbs 20 calories of heat (assume not during a state change), the resulting water temperature change is - 272.975 °C .
Calculation ,
Formula used : Q = m × Cp × change in temperature
Q = m × Cp × ΔT ( i )
Where Q is the heat supplied in calories = 20 cal ( given )
m is the mass of water in gram = 2 g ( given )
Cp is the specific heat capacity of water at constant pressure = 1 cal / gK ( known )
Thus , after putting the value of heat supplied in calories , mass of water and specific heat capacity of water in equation ( i ) we get the value of change in temperature .
1 cal / gK = 2 g × 20 cal × ΔT
ΔT = 1 cal / gK / 2 g × 20 cal = 0.025 K
ΔT = 0.025 K - 273 = - 272.975 °C
To learn more about water temperature
https://brainly.com/question/26636802
#SPJ4
for the following reaction, 5.42 grams of sodium iodide are mixed with excess chlorine gas. the reaction yields 1.76 grams of sodium chloride. chlorine (g) sodium iodide (s) sodium chloride (s) iodine (s) what is the theoretical yield of sodium chloride ? grams what is the percent yield of sodium chloride ? %
Answers
The sentence makes it clear that none of these are true.
What purpose does sodium iodide serve?Iodine shortage is treated or prevented using sodium iodide. Iodine is required by the body in proper development and growth. Potassium iodide may be required for people who need additional iodine or cannot acquire adequate copper from their normal diet. Your thyroid gland needs iodine to operate correctly.
Which class of medication is sodium iodide?A complement used in complete parenteral feeding is sodium iodide. A water-soluble ionic bond having a crystal lattice is sodium iodide. Iodine can be obtained from sodium iodide, which can be given as a supplement for complete parenteral nutrition but is most frequently utilized in
To know more about sodium iodide visit;
https://brainly.com/question/19952754
#SPJ4
The volume of container 2 i 27. 32 L. How many mole of the ga are in container 2?
Answers
The number of moles in container 2 is 33.3moles when the container has 27.32L of gas inside it
The number of moles of gas in container 2 can be calculated using the Ideal Gas Law:
n = PV/RT
where n is the number of moles of the gas with known volume,
P is the pressure (assumed to be 1 atm for ideal gases),
V is the volume (27.32 liters),
R is the ideal gas constant (0.0821 L·atm/mol·K) and
T is the temperature (assumed to be 273.15 K).
Plugging in the values, we get:
n = (1 atm)(27.32 L)/(0.0821 L·atm/mol·K)(273.15 K)
n = 33.3 mol
Therefore, there are 33.3 moles of gas in container 2.
To know more about gas laws in chemistry, click below:
https://brainly.com/question/25290815
#SPJ4
g does fission of light or heavy elements produce the most energy per mole of the given isotope? explain by discussing mass defect and nuclear binding energy.
Answers
Depending on the element's atomic number, fusion provides significantly more energy for lighter elements than fission does for heavier ones.
The term "binding energy" is the key to comprehending this. The combined mass of protons and neutrons in a nucleus, when they are held together by the strong force, is always less than the combined mass of the same number of unbound protons and neutrons.
The mass disparity is transformed into energy when two light nuclei combine to form a heavier nucleus. This means that to cause the nucleus to disintegrate, the same amount of energy must be added.
The issue is that a lot of energy is released when hydrogen and helium are fused together. Less energy is released when heavier elements are fused.
For more information on fission kindly visit to
https://brainly.com/question/7720206
#SPJ4