In the second experiment, bacteria are grown in a culture medium depleted of Na+. Under these conditions, when P3 is inactivated and a drug stimulates the activity of P1, the pH of the culture medium decreases unless a chelating agent (trapping Na+) is added to the culture medium.
Complete the following sentences. (2 attempts)
From the second experiment you conclude that in the presence of substrate S, the ___(A)___ results in the P1-dependent ____(B)____ of Na+ outside the bacteria. The Na+ ____(C)____ via P2 and this transport is coupled with H+ ___(D)___ leading to the formation of a proton motive force/Na+ gradient. The extracellular ___(E)___ re-enters the cell via P3 leading to ATP synthesis.
Options:
A: decarboxylation, carboxylation
B: export, import
C: re-enters, exits
D: export, import
E: H+, Na+
Answers
In the second experiment, bacteria are grown in a culture medium depleted of Na+. the culture medium is the gel or the liquid contains the nutrients and it is used to grow the bacteria.
In the experiment, The bacteria is grown in the culture medium. we conclude that , in the presence of the substrate S, the decarboxylation results in the P1 - dependent export of the Na⁺ outside the bacteria. The Na⁺ re-enters via P2 and this transport is coupled with the H⁺ exports leading to the formation of the proton motive force/ Na⁺ gradient.
The extracellular H⁺ reenters the cell via P3 leading to the ATP synthesis.
To learn more about culture medium here
https://brainly.com/question/14932618
#SPJ4
The second experiment involves the growth of bacteria in a medium that lacks Na+. The liquid or gel used as the culture medium includes nutrients and is used to cultivate microorganisms.
The bacteria is cultivated in the experiment's culture medium. We come to the conclusion that the decarboxylation causes the Na+ to be exported from the bacteria in a P1-dependent manner when the substrate S is present. The proton motive force/Na+ gradient is created when the Na+ re-enters through P2 and is transported together with the H+ exports. Through P3, extracellular H+ returns to the cell and triggers the creation of ATP.
Learn more about decarboxylation here-
https://brainly.com/question/29573197
#SPJ4
Related Questions
A 25.0 g sample of warm water at 40.0⁰C was added to a 25.0 g sample of water in a Styrofoam coffee cup calorimeter initially at 20.0⁰C. The final temperature of the mixed water and calorimeter was 29.5⁰C. Calculate the heat capacity of the coffee cup calorimeter. The specific heat of water is 4.184 J/g∙⁰C.
a.
0.189 J/⁰C
b.
27.3 J/⁰C
c.
11.0 J/⁰C
d.
116 J/⁰C
Answers
Answer:
2024.70 J
Explanation:
The heat capacity of the coffee cup calorimeter can be calculated using the following formula:
q_calorimeter = q_water + q_water_final
where q_calorimeter is the heat absorbed by the coffee cup calorimeter, q_water is the heat lost by the warm water, and q_water_final is the heat gained by the cold water.
First, calculate q_water:
q_water = m_water * c_water * ΔT
where m_water = 25.0 g is the mass of the warm water, c_water = 4.184 J/g°C is the specific heat of water, and ΔT = (40.0°C - 29.5°C) = 10.5°C is the change in temperature.
q_water = 25.0 g * 4.184 J/g°C * 10.5°C = 1057.35 J
Next, calculate q_water_final:
q_water_final = m_water * c_water * ΔT
where m_water = 25.0 g is the mass of the cold water, c_water = 4.184 J/g°C is the specific heat of water, and ΔT = (29.5°C - 20.0°C) = 9.5°C is the change in temperature.
q_water_final = 25.0 g * 4.184 J/g°C * 9.5°C = 967.35 J
Finally, calculate the heat capacity of the coffee cup calorimeter:
q_calorimeter = q_water + q_water_final = 1057.35 J + 967.35 J = 2024.70 J
So the heat capacity of the coffee cup calorimeter is 2024.70 J.
A 25.0 g sample of warm water at 40.0⁰C was added to a 25.0 g sample of water in a Styrofoam coffee cup calorimeter initially at 20.0⁰C. 2024.70 J is the heat capacity of the coffee cup calorimeter.
What is heat capacity?A physical feature of matter known as heat capacity and thermal capacity is the quantity of heat that must be applied to an object in order to cause a unit change in temperature. Heat capacity is measured in joules per kelvin (J/K), the SI unit. A broad property is heat capacity.
The particular heat capacity, which can be calculated by dividing an object's heat capacity by its mass, is the comparable intense attribute. The molar heat capacity is obtained through dividing the specific heat even by molecular weight of the substance. The heat capacity per volume is gauged by the volumetric heat capacity. The term "thermal mass" is frequently used in civil engineering and architecture to describe a building's ability to hold heat.
q calorimeter = q water + q water final
q water = m ×c water ×ΔT
q water = 25.0 g×4.184 J/g°C ×10.5°C
= 1057.35 J
q water final = m×c of water × ΔT
q water final = 25.0 g×4.184 J/g°C ×9.5°C
= 967.35 J
q calorimeter = q water + q water final
= 1057.35 J + 967.35 J
= 2024.70 J
Therefore, 2024.70 J is the heat capacity of the coffee cup calorimeter.
To know more about heat capacity, here:
https://brainly.com/question/29766819
#SPJ2
What is the new molarity if 55.0mL of water is added 25.0mL of 0.119M
NaCl solution.
Answers
Answer: 0.04M.
Explanation: Using M1V1=M2V2, we can find the new molarity (M2).
We are given M1 and V1 with 0.119M and 25mL, we are also given V2 since it says we added 55mL to the original 25mL.
25mL+55mL = 80mL.
Let's convert mL to L by dividing by 1000.
V1 = 25ml/1000 = 0.025L
V2 = 80mL/1000 = 0.08L.
Now plug in and solve algebraically.
0.119 * 0.025 = M2 * 0.08. Divide both sides by 0.08.
\(\frac{0.119*0.025}{0.08}\) = 0.037.
M2 = 0.037M
However, since we are given 25mL which only has 2 sig figs, the new molarity os 0.04M.
What volume will 5.00 mol of an ideal gas occupy at 25.0 C. and 153 kPa of pressure?
Answers
Answer:
5.00 mol of an ideal gas will occupy 103.6 L at 25.0 C and 153 kPa of pressure.
Explanation:
Using the ideal gas law, PV=nRT, where P is the pressure, V is the volume, n is the number of moles of gas, R is the gas constant, and T is the temperature in Kelvin, we can solve for V.
First, we need to convert the temperature from Celsius to Kelvin by adding 273.15 K. Therefore, the temperature is 25.0 + 273.15 = 298.15 K.
Next, we can plug in the values we know:
PV = nRT
(153 kPa) V = (5.00 mol) (8.31 J/mol*K) (298.15 K)
Simplifying:
V = (5.00 mol) (8.31 J/mol*K) (298.15 K) / (153 kPa)
V = 103.6 L
Therefore, 5.00 mol of an ideal gas will occupy 103.6 L at 25.0 C and 153 kPa of pressure.
For a gaseous reaction, standard conditions are 298 K and a partial pressure of 1 atm for all species.
For the reaction
N2(g)+3H2(g)↽−−⇀2NH3(g)
the standard change in Gibbs free energy is Δ°=−32.8 kJ/mol
. What is ΔG for this reaction at 298 K when the partial pressures are N2=0.350 atm
, H2=0.300 atm
, and NH3=0.750 atm
?

Answers
The ΔG for the reaction at 298 K and the given partial pressures is -55.53 kJ/mol.
What is ΔG ?
The Gibbs free energy change for a reaction under non-standard conditions can be calculated using the following equation:
ΔG = ΔG° + RTln(Q)
where ΔG is the Gibbs free energy change, ΔG° is the standard Gibbs free energy change, R is the gas constant (8.314 J/(mol·K)), T is the temperature in kelvin, and Q is the reaction quotient.
The reaction quotient, Q, can be calculated using the partial pressures of the gases involved in the reaction:
Q = (P(NH3))² / (P(N2) x P(H2)³)
Plugging in the given values, we get:
Q = (0.75 atm)² / (0.35 atm x 0.30 atm³) = 0.2667
Now we can calculate the ΔG for the reaction:
ΔG = ΔG° + RTln(Q)
ΔG = (-32.8 kJ/mol) + (8.314 J/(mol·K) x 298 K x ln(0.2667))
ΔG = -32.8 kJ/mol + (-22.73 kJ/mol)
ΔG = -55.53 kJ/mol
Therefore, the ΔG for the reaction at 298 K and the given partial pressures is -55.53 kJ/mol.
What is reaction quotient?
Reaction quotient, commonly denoted as Q, is a measure of the relative concentrations of reactants and products in a chemical reaction at a particular moment in time. It is calculated by dividing the concentration of the products raised to their stoichiometric coefficients by the concentration of the reactants raised to their stoichiometric coefficients.
The equation for the reaction quotient Q is similar to the equilibrium constant Kc, but with the concentrations of the reactants and products at any time during the reaction, rather than at equilibrium. When the reaction is at equilibrium, the reaction quotient is equal to the equilibrium constant.
To know more about ΔG, visit:
https://brainly.com/question/13961239
#SPJ1
A sample of a gas occupies 2016 mL at 83°C
and 740 torr. At what temperature would
it occupy 1776.4 mL if the pressure is kept constant?
Answer in units of °C.
Answers
A sample of a gas occupies 2016 mL at 83°C and 740 torr. At 196.5°C, it occupy 1776.4 mL if the pressure is kept constant.
What is temperature?The physical concept of temperature indicates in numerical form how hot or cold something is. A thermometer is used to determine temperature. Thermometers are calibrated using a variety of temperature scales, which historically defined distinct reference points or thermometric substances.
The most popular scales include the Celsius scale, sometimes known as centigrade, with the unit symbol °C, the scale for Fahrenheit (°F), or the Kelvin scale (K), with the latter being mostly used for scientific purposes. Another of the seven foundational units of the Worldwide System of Units is the kelvin.
T2 = (T1V2)/V1
T2 = (237 x1776.4)/ 2016
T2 = 196.5°C
Therefore, 196.5°C is the temperature.
To know more about temperature, here:
https://brainly.com/question/23411503
#SPJ2
how many molecules are in 0.610 moles of neon gas?
Answers
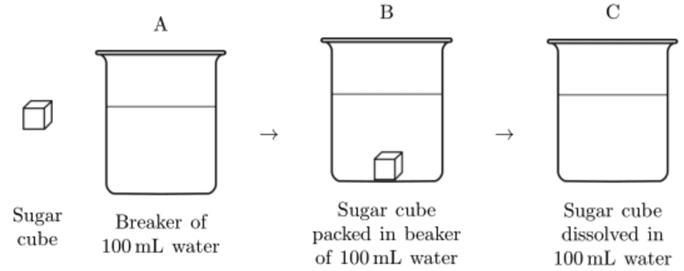
A sugar cube was placed into a beaker containing 100 mL of water at room temperature and completely dissolved into the water. This process is represented by the series of diagrams labeled A, B, and C below. Describe one way that the dissolved sugar at C could be separated from the water.
Answers
Answer:
Boil the water until it evaporates
Explanation:
If the water evaporates the sugar will no longer bond to it and then percipitate at the bottom of the beaker.
Arrange the following elements from greatest to least tendency to accept an electron. Rank from greatest to least tendency to accept an electron. To rank items as equivalent, overlap them. Br Se Ge Ca K I arranged this in the order i listed above and was told it was the incorrect answer and Your answer shows the general trend, but there are some exceptions. For example, the tendency to gain an electron is lower than expected if a stable electron configuration is lost.
Answers
Answer:
Br > Se > Ge > K> Ca
Explanation:
Generally in the periodic table, we know that electron affinity increases across a period. Electron affinity refers to the energy released when an extra electron is added to a neutral gaseous atom. Most times, the addition of an electron leads to the formation of a uninegative ion.
The sequence of decrease in electro affinity values appears to be consistent until we get to calcium and potassium. One would have ordinarily expected potassium to have a lower electron affinity than calcium. However, if potassium accepts an electron, that electron goes on to fill the 4s level. This process is more exothermic than in the case of calcium where the extra electron is added to the 3d level. The energy difference between the 4s and 3d levels is enough to make the process less exothermic (only 2.37KJmol-1) compared to that of potassium (48.4 KJmol-1). This explains the order of arrangement seen in the answer above.
If the atom is assumed to be a sphere, what is the volume in cm^3 of a single Au atom?
Answers
Answer:
The equation for a volume of a sphere is V= (4/3)πr3
The above equations says we need to find the radius of our sphere. According to https://www.ptable.com/ the radius of a gold atom is 144 pm.
The questions wants the answer to be in cm3 which means we need to convert picometers into centimeters. 1cm= 1010pm
144pm x (1cm/1010pm) = 1.44 x 10-8cm
Now we plug the radius of the gold atom into the volume equation.
V= (4/3)π(1.44x 10-8cm)3 -----> Answer= 1.25x10-23 cm3
HOPE IT'S HELPS YOUConsider the following neutral electron configurations in which n has a constant value. Which configuration would belong to the element with the most negative electron affinity, E-ea?
a. ns^2
b. ns^2 np^2
c. ns^2 np^5
d. ns^2 np^6
Answers
Answer:
ns^2 np^5
Explanation:
Now let us try to analyse our options carefully in order to make an informed decision about the correct answer.
The answer cannot be ns^2 since any atom with this configuration must be a metal in group 2. Secondly, ns^2 np^2 is an element in group 14. Again, this specie cannot have the most negative electron affinity. The atom whose outermost electron configuration is ns^2 np^6 must be a noble gas since the outermost shell possesses eight electrons. This cannot be the answer we are seeking for.
However, an outermost electron configuration of ns^2 np^5 will apply to a halogen. Halogens have a very negative electron affinity. Hence a specie with this electron configuration must have the most negative electron affinity among the options.
The correct answer to the question is Option C. ns² np⁵
Let n represent the valence shell.
NOTE: Valence shell is the outermost shell of an atom.
Thus, we shall determine the valence electrons for each options given in the question.
For Option AValence shell = ns²
Valence electron = 2For Option BValence shell = ns²np²
Valence electron = 2 + 2
Valence electron = 4For Option CValence shell = ns²np⁵
Valence electron = 2 + 5
Valence electron = 7For Option DValence shell = ns²np⁶
Valence electron = 2 + 6
Valence electron = 8Electronegativity is the tendency of an atom to attract electron(s). Atoms with more valence electrons tends to attract electrons more. From the illustraton above, we can see that Option C has 7 valence electrons while Option D has 8 valence Option D shows that the atom has completely filled outermost shell and as such, it does not attract electron(s) Option C has the highest valence electron (i.e 7) besides option D which do not attract electrons. Therefore, atom with 7 valence electrons will have a higher electronegativity.Therefore, Option C. ns² np⁵ gives the correct answer to the question.
Learn more: https://brainly.com/question/10531792
n today's experiment, Solutions A and B are prepared as follows. Solution A: Solution B: 2.0 mL of 3.00 x 10-4 M bromcresol green 2.0 mL of 3.00 x 10-4 M bromcresol green 5.0 mL of 1.60 M acetic acid (HAc) 2.0 mL of 0.160 M sodium acetate (NaAc) 2.0 mL of 0.200 M KCl diluted to a total volume of 50 mL diluted to a total volume of 50 mL How many mL of Solution A must be added to Solution B to give a buffer that is equimolar in HAc and Ac-
Answers
Answer:
2 mL of Solution A must be added to Solution B to give a buffer that is equimolar.
Explanation:
Given the data in the question;
First we determine the number of sodium acetate;
⇒ molarity × volume ( L )
⇒ 0.16 × 2.0 mL
⇒ 0.16 × 0.002 L
⇒ 0.00032
Now, Molarity of sodium acetate = moles / Volume(L)
⇒ ( 0.00032 / 50 ) × 1000
⇒ 0.0064
Since number of moles of acetic acid that should be added tp make equimolar solution is 0.00032
and Molarity of acetic acid is 0.16 molL⁻¹
Let X represent the volume that should be added.
so;
Molarity = Moles / Volume (L)
we substitute
0.16 = (0.00032 / X) × 1000
0.16 = 32 / X
X = 0.32 / 0.16
X = 2 mL
Therefore, 2 mL of Solution A must be added to Solution B to give a buffer that is equimolar.
An experiment is set up with a glass bottle and a candle. The starting volume of the bottle is 250 mL at a pressure of 1 atm. The bottle contains 0.00600 moles of O2 gas. A candle is then put into the bottle and the flame consumes most of the oxygen. The temperature is 70°C. After the candle goes out, there is only 0.000540 moles of oxygen remaining and the temperature drops to 35°C. Assume the volume is still 250 mL. What is the pressure inside the bottle?
________atm (use 3 sig figs with one zero in front of the decimal)
30 points
Answers
Answer: 0.112 atm.
Explanation:
(PV = nRT), which states that the pressure times volume of a gas is equal to the number of moles of gas times the ideal gas constant times the temperature.
Since the volume of the bottle is constant, we can rearrange the Ideal Gas Law to solve for pressure:
P = (nRT) / V
where
n = 0.000540 moles (the amount of O2 remaining after the candle goes out)
R = 8.31 J/mol*K (the ideal gas constant)
T = 35 + 273 = 308 K (the temperature in kelvins)
V = 250 mL = 0.25 L (the volume of the bottle in liters)
Plugging in the values, we get:
P = (0.000540 * 8.31 * 308) / 0.25
P = 0.112 atm
So, the pressure inside the bottle after the candle goes out is approximately 0.112 atm.
Why aren’t two hydrogen atoms bonded together considered a compound? Explain.
Answers
Answer:
Hydrogen gas (H2) is a molecule, but not a compound because it is made of only one element. Water (H2O) can be called a molecule or a compound because it is made of hydrogen (H) and oxygen (O) atoms. There are two main types of chemical bonds that hold atoms together: covalent and ionic/electrovalent bonds.
Write an equation for the neutralization of lemon juice with baking soda. Your equation should show a proton transfer to form carbonic acid and a salt.
Answers
The equation of the neutralization reaction is:
HOC(CO₂H)(CH₂CO₂H)₂ + Na₂CO₃ ----> HOC(CO₂Na)(CH₂CO₂Na)₂ + H₂O
What is the acid in lemon juice?The main acid in lemon juice is citric acid.
Citric acid is a tricarboxylic acid and will react with baking soda to form a salt and water.
The equation of the neutralization reaction is shown below:
HOC(CO₂H)(CH₂CO₂H)₂ + Na₂CO₃ ----> HOC(CO₂Na)(CH₂CO₂Na)₂ + H₂O
In conclusion, the neutralization of citric acid in lemon juice produces a salt and water.
Learn more about neutralization reaction at: https://brainly.com/question/15042730
#SPJ1
Part A
A solution of cough syrup contains 5.00% active ingredient by volume. If the total volume of the bottle is 68.0 mL, how many milliliters of ac
ingredient are in the bottle?
Express your answer with the appropriate units.
Answers
There are 3.4 milliliters of the active ingredient in the cough syrup bottle.
To calculate the volume of the active ingredient in the cough syrup bottle, we need to multiply the total volume of the bottle by the percentage of the active ingredient.
Given:
Total volume of the bottle = 68.0 mL
Percentage of active ingredient = 5.00%
First, we convert the percentage to a decimal by dividing it by 100:
Percentage of active ingredient = 5.00% = 5.00/100 = 0.05
Next, we calculate the volume of the active ingredient:Volume of active ingredient = Total volume of the bottle × Percentage of active ingredient
Volume of active ingredient = 68.0 mL × 0.05
Volume of active ingredient = 3.4 mL
Therefore, there are 3.4 milliliters of the active ingredient in the cough syrup bottle.
It's important to note that the calculation assumes a homogeneous distribution of the active ingredient throughout the solution.
For more such questions on cough syrup visit:
https://brainly.com/question/2970281
#SPJ8
write the structural formula for 2-bromo-3-chloro-4,4-dimethylpentanal
Answers
Answer:
Br-CH2-CH(CH3)2-C(Cl)H-CH(CH3)2-CHO
Explanation:
The molecule has a total of 14 carbon atoms, 13 hydrogen atoms, and 1 bromine atom. The carbon atoms are arranged in a chain with a methyl group attached to the second carbon atom, a chlorine atom attached to the third carbon atom, and two methyl groups attached to the fourth carbon atom. The fifth carbon atom has a carbonyl group attached to it.
The molecule is an aldehyde, which means that it has a carbonyl group (C=O) at the end of the chain. The carbonyl group is polar, and the oxygen atom has a partial negative charge. The hydrogen atom has a partial positive charge. This polarity makes the aldehyde group susceptible to nucleophilic attack.
The bromine and chlorine atoms are both electrophilic, which means that they have a partial positive charge. This makes them susceptible to nucleophilic attack.
The methyl groups are non-polar and do not have any significant reactivity.
The molecule is a chiral molecule, which means that it has a mirror image that is not superimposable on itself. This is because the carbon atom with the carbonyl group is attached to four different groups.
The molecule is a liquid at room temperature and has a strong odor. It is used in a variety of products, including perfumes, flavorings, and plastics.
Example: One liter of saturated calcium fluoride
solution contains 0.0167 gram of CaFat 25°C.
Calculate the molar solubility of, and Ksp for, CaF2.
Answers
Answer:
\(Molar\ solubility=2.14x10^{-4}M\)
\(Ksp=3.91x10^{-11}\)
Explanation:
Hello,
In this case, given that 0.0167 grams of calcium fluoride in 1 L of solution form a saturated one, we can notice it is the solubility, therefore, the molar solubility is computed by using the molar mass of calcium fluoride (78.1 g/mol):
\(Molar\ solubility=\frac{0.0167gCaF_2}{1L}*\frac{1molCaF_2}{78.1gCaF_2} \\\\Molar\ solubility=2.14x10^{-4}M\)
Next, since dissociation equation for calcium fluoride is:
\(CaF_2(s)\rightarrow Ca^{2+}(aq)+2F^-(aq)\)
The equilibrium expression is:
\(Ksp=[Ca^{2+}][F^-]^2\)
We can compute the solubility product by remembering that the concentration of both calcium and fluoride ions equals the molar solubility, thereby:
\(Ksp=(2.14x10^{-4})(2*2.14x10^{-4})^2\\\\Ksp=3.91x10^{-11}\)
Regards.
meg goes swimming on a hot afternoon. When she comes out the pool her foot senses that the pavement is unbearably hot. Suppose meg wants to apply the scientific method to discover reasons for the hot pavement. What is the next step she should take?
A. Analyze the data
B. Ask questions
C. communicate the results
D. Make observations
Answers
The next step Meg should take in applying the scientific method to discover the reasons for the hot pavement is B. Ask questions. Option B
Asking questions is a crucial step in the scientific method because it allows for the formulation of a hypothesis and the design of experiments to test that hypothesis. By asking questions, Meg can begin to explore the possible factors contributing to the hot pavement and formulate hypotheses to explain the phenomenon.
In this case, Meg can ask questions such as:
Why is the pavement so hot?
Does the pavement always feel hot after swimming?
Is the temperature of the pavement affected by the weather conditions?
Are there specific materials or colors used in the pavement that may contribute to its heat absorption?
These questions will help guide Meg in her investigation and provide a starting point for gathering more information and designing experiments to test her hypotheses.
Once Meg has formulated her questions, she can move on to the next steps of the scientific method, which include making observations, analyzing data, conducting experiments, and communicating the results.
These subsequent steps will allow her to gather data, analyze it, and draw conclusions based on evidence, leading to a better understanding of the factors contributing to the hot pavement. Meg can then communicate her findings to others, furthering scientific knowledge and potentially finding solutions to mitigate the problem.
Option B
For more such questions on scientific method visit:
https://brainly.com/question/28228027
#SPJ8
Metamorphism can best be defined as
a. Compaction and cementation of rock fragments
O
b. Precipitation of minerals dissolved in water
c. Solidification of magma by cooling
d. Changing of a rock by heat and pressure
Need help ASAP
Answers
Answer:
The answer is D if it's wrong let me know pls
Someone plz hello me ASAP it would be appreciated on question 7 btw

Answers
Answer:
Kinetic Energy is the correct Answer.
Explanation:
At the highest point on the roller coaster (assuming it has no velocity), the object has a maximum quantity of gravitational potential energy. As the object begins moving down to the bottom, its gravitational potential energy begins to decrease and the Kinetic Energy starts to increase.
At what speed must a neutron travel to have a wavelength of 25.0 pm? The mass of a neutron is 1.67x10^-27 kg.
Answers
Speed must a neutron travel to have a wavelength of 25.0 pm.The mass of a neutron is 1.67x10^-27 kg is 3.17 × 10³ m/s.
Given that :
mass of neutron = 1.67 × 10⁻²⁷ Kg
wavelength = 25 pm = 2.5 × 10⁻¹¹m
h = 6.63 × 10⁻³⁴ Js ( 1 J = Kg m /s² )
using debroglie,s wavelength , we get
λ = h / mv
v = h / mλ
v = 6.63 × 10⁻³⁴ / ( 2.5 × 10⁻¹¹ × 1.67 × 10⁻²⁷ )
v = 6.63 × 10⁻³⁴ J.s / 20.87 × 10⁻³⁸
v = 3.17 × 10³ m/s
Thus, Speed must a neutron travel to have a wavelength of 25.0 pm.The mass of a neutron is 1.67x10^-27 kg is 3.17 × 10³ m/s.
To learn more about wavelength here
https://brainly.com/question/28762766
#SPJ1
What is Na2Co3? How look like that's?
Answers
Sodium carbonate, often referred to as Na2CO3, is a chemical compound composed of atoms of sodium (Na), carbon (C) and oxygen (O).
It is also sometimes called washing soda or soda ash. At room temperature, sodium carbonate is a white, crystalline solid that is very soluble in water. According to the chemical formula of the sodium carbonate molecule, Na2CO3, each molecule consists of two sodium atoms (Na), one carbon atom (C) and three oxygen atoms (O). The atomic configuration in sodium carbonate is shown in the given diagram.
A trigonal planar arrangement is formed when the central carbon atom is bonded to three oxygen atoms. The structure of sodium carbonate is completed by two sodium atoms joined to oxygen atoms.
Learn more about Sodium carbonate, here:
https://brainly.com/question/31422792
#SPJ1

If 100 ml of a 0.75 m hno3 is required to exactly neutralize 50 ml of naoh what is the concentration of the base?
Answers
Answer:
1.5M
Explanation:
M1V1=M2V2
(0.75M)(100ml)=(M2)(50 ml)
M2= 1.5 M
*Text me at 561-400-5105 for private tutoring if interested: I can do homework, labs, and other assignments :)
hhhhhheeeeeellllllppppp me ffffaaaßstttt ananaanaanswswwwwweeeerrrr
what are the common diseases that are
found in poultry ?
Answers
Answer:
Diseases of Poultry
a. ESCHERICHIA COLI INFECTIONS.
b. SALMONELLOSES.
c. PARATYPHOID INFECTIONS.
d. FOWL CHOLERA.
e. RIEMERELLA ANATIPESTIFER INFECTIONS.MYCOPLASMA.
f. NECROTIC ENTERITIS.
g. CHOLANGIOHEPATITIS IN BROILER CHICKENS.
Explanation:
Answer:
◎Bacterial diseases
◎Mycoplasmosis (CRD, Air sac, Sinusitis)
◎Fowl Cholera
◎Necrotic Enteritis
◎Ulcerative Enteritis (Quail disease)
◎Pullorum Disease
◎Fowl
◎Botulism
◎Infectious Coryza
◎Omphalitis
◎Erysipelas
◎Parasitic diseases (internal)
◎Parasitic diseases (external)
◎Infectious Bronchitis
◎Viral diseases
◎Newcastle Disease
◎Quail Bronchitis
◎Lymphoid Leukosis
◎Marek's Disease (Visceral Leukosis)
◎Infectious Bursal Disease (Gumboro)
Explanation:
:)
Hi do you know this?

Answers
Answer:
2
Explanation
It seems logical
What is the potential energy of a 250 kg diver standing on a platform 7 meters high?
Answers
Answer:
17150 Joules
Explanation:
Mass=250 kg
Meters=7 meters high
Multiply mass, meters, and the gravity constant 9.8 meters/second to get the potential energy in joules:
250 * 7 * 9.8 =
1750 * 9.8 = 17150 Joules
To learn more about potential energy, click here:
https://brainly.com/question/20161995
How many L of 4.0 M solution can be made with 132g of NaCI ?
A)9.02L
B)9.02ml
C)0.9L
Answers
Answer:
0.430 Litre
Explanation:
The first thing that you need to do here is to convert the mass of lithium bromide to moles by using the compound's molar mass.\(100g \frac{1 moleNaCl}{58.5g} = 1.71 moles of NaCl\)
Now, the molarity of the solution is simply a measure of the number of moles of solute, which in your case is NaCl, present for every 1.00 L of the solution.
In order to have a 4.00-M solution of NaCl, you need to have 4.00 moles of NaCl for every 1.00 L of this solution.
You know that your sample contains 1.71 moles of NaCl, so you can use the molarity of the solution as a conversion factor to determine how many liters of this solution can be made.
\(1.71 mole NaCl \frac{ 1 L Solution}{4 mole NaCl} = 0.43 L solution\).
Describe the advantages of the hydrogen-rich fuel cell when compared to the conventional electrochemical cells such as lead-acid battery. (4)
Answers
The hydrogen-rich fuel cell offers advantages in terms of efficiency, environmental impact, operating time, refueling speed, weight, size, and lifespan when compared to conventional electrochemical cells like the lead-acid battery.
The hydrogen-rich fuel cell offers several advantages over conventional electrochemical cells like the lead-acid battery. Here are some of the key advantages:
1. Higher Efficiency: Hydrogen fuel cells have higher energy conversion efficiencies compared to lead-acid batteries. Fuel cells can convert chemical energy directly into electrical energy with minimal loss, while lead-acid batteries have inherent energy losses due to factors such as internal resistance and heat dissipation.
2. Clean and Environmentally Friendly: Hydrogen fuel cells produce electricity through the reaction of hydrogen and oxygen, with water being the only byproduct. They do not produce harmful emissions or contribute to air pollution, making them a cleaner and more sustainable power source compared to lead-acid batteries, which require the use of chemicals like sulfuric acid.
3. Longer Operating Time: Fuel cells have longer operating times compared to lead-acid batteries. Lead-acid batteries have a limited capacity and need to be recharged frequently, while fuel cells can continuously generate electricity as long as there is a supply of hydrogen.
4. Faster Refueling: Refueling a fuel cell is faster compared to recharging a lead-acid battery. Fuel cells can be refueled by replenishing the hydrogen supply, which can be done relatively quickly. In contrast, lead-acid batteries require a longer time to recharge, typically hours, depending on the battery's capacity and charging rate.
5. Lighter Weight and Compact Size: Hydrogen fuel cells have a higher energy density compared to lead-acid batteries, meaning they can store more energy in a smaller and lighter package. This makes fuel cells more suitable for applications where weight and space are critical, such as in portable devices or electric vehicles.
6. Longer Lifespan: Fuel cells generally have a longer lifespan compared to lead-acid batteries. Lead-acid batteries can experience degradation over time due to factors like sulfation, which can reduce their overall capacity and lifespan. Fuel cells, on the other hand, can provide consistent performance over an extended period with proper maintenance.
These advantages make fuel cells a promising technology for various applications, including transportation, stationary power generation, and portable electronics.
for more questions on fuel cell
https://brainly.com/question/14122421
#SPJ8
Please show you work
This is 7th grade science

Answers
2) It will accelerate to the right 8>6
3) It will not move. 15=15
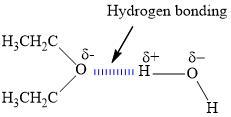
2. How many hydrogen bonds can form between a single ether molecule and water molecules? Draw the structures to explain.
Answers
Ether can only form one hydrogen bond per molecule.
What is hydrogen bonding?We know that the hydrogen bond is the kind of bond that occurs when the dipole of water interacts with the dipole that is on another molecule. We can see this in a lot of hydrides of electronegative elements.
We can see that ether has only one electronegative atom and that is oxygen from the image that is shown in the answer. This oxygen atom can interact with the positive end of the dipole in only one water molecule at a time.
Learn more about hydrogen bonding:https://brainly.com/question/15099999
#SPJ1