Answers
Answer:
2.62*10^-3
Explanation:
To do this by hand, you need to understand the rules of scientific notation.
Answer:
0.00262 in scientific notation is 2.62 x 10^-3
Related Questions
HELP !!
Question 5 Multiple Choice Worth 3 points)
(01.04 LC)
Which of the following is not an intensive physical property?
Magnetism
Boiling point
Thermal conductivity
Volume
Answers
It would be volume.
Volume is not an intensive property because it does change as the amount of substance increases or decreases. The rest of the properties are constant no matter the amount of substance.
Answer:
Volume
Look at the guy above me, okay-- they're smarter then me-
Dau coroana va roggg!!!!!!

Answers
Answer:
it's very easy and simple answer u can't do it
How many calories of energy are found in 8.3 grams or Fat? Every gram of fat
can produce 38 KJ of energy.
Answers
Answer: asd asd asd asdasdasdasd
Dominic made the table below to organize his notes about mixtures. A 1-column table. The first column labeled properties of mixtures has entries has no set composition, must have more than one state of matter, must have more than one substance. What mistake did Dominic make? The title should read “Properties of Solutions” because some mixtures do not have all of the properties listed. There is a definite recipe to make each mixture, so the composition of a mixture is set. Although it is possible to have more than one state, it is also possible to have only one state. A single substance can be used to make a mixture if the substance is composed of more than one element.
Answers
Answer:
Exxplanation:
Answer:
Although it is possible to have more than one state, it is also possible to have only one state.
Explanation:
A photon has a frequency of 5.40 × 10^4 Hz. Calculate the energy (in joules) of 1 mole of photons with this frequency. Enter your answer in scientific notation.
Answers
The energy (in joules) of 1 mole of photons with this frequency is 1.99 x 10⁻¹⁴ J per photon.
What is photon ?Should a substance happen to have a lot of electrons in a higher level, and a lower level is mostly empty then a photon can cause an electron to transfer from a higher state to a lower one. This change releases energy and creates a new photon, in addition to the one which caused the transfer. This photon can in turn induce more electrons to fall to a lower state.
use formula
The energy of 1 mole of photons with the given frequency can be calculated using the following equation:
Energy (J) = Avogadro's number x Plank's Constant x Frequency
Therefore,
Energy (J) = 6.02 x 10²³ x 6.626 x 10⁻³⁴ x 5.40 x 10⁴
Energy (J) = 1.99 x 10⁻¹⁴ J
Therefore, the energy of 1 mole of photons with a frequency of 5.40 x 10⁴ Hz is 1.99 x 10⁻¹⁴ J, expressed in scientific notation.
To know more about photon , visit ;
brainly.com/question/20912241
#SPJ1
What are 2 comparisons between organisms and music?
Help me fast I put 98 points pls help me no links I am serious.
3rd repost
Answers
The composition of a compound is 28.73% K, 1.48% H, 22.76% P, and 47.03% O. The molar mass of the
compound is 136.1 g/mol.
I
Answers
The compound has an empirical formula of \(K_2H_2P_2O_8\) and a molecular formula of \(K_2HPO_4\).
The given compound has a percent composition of K = 28.73%, H = 1.48%, P = 22.76%, and O = 47.03%. Its molar mass is 136.1 g/mol. To determine its molecular formula, we need to find its empirical formula and calculate its molecular formula from its empirical formula.The empirical formula is the smallest whole number ratio of atoms in a compound. It can be determined by converting the percent composition of the elements into their respective moles and dividing each by the smallest number of moles calculated. The moles of K, H, P, and O in 100 g of the compound are: K = 28.73 g x (1 mol/39.1 g) = 0.734 molH = 1.48 g x (1 mol/1.01 g) = 1.46 molP = 22.76 g x (1 mol/30.97 g) = 0.736 molO = 47.03 g x (1 mol/16.00 g) = 2.94 molDividing each by the smallest number of moles gives the following ratios: K = 0.734/0.734 = 1H = 1.46/0.734 = 2P = 0.736/0.734 = 1.002O = 2.94/0.734 = 4. The empirical formula of the compound is \(K_2H_2P_2O_8\). To calculate the molecular formula, we need to determine the factor by which the empirical formula should be multiplied to obtain the molecular formula. This can be done by comparing the molar mass of the empirical formula to the molar mass of the compound.The molar mass of \(K_2H_2P_2O_8\) is: \(M(K_2H_2P_2O_8)\) = (2 x 39.1 g/mol) + (2 x 1.01 g/mol) + (2 x 30.97 g/mol) + (8 x 16.00 g/mol) = 276.2 g/mol. The factor by which the empirical formula should be multiplied is: M(molecular formula)/M(empirical formula) = 136.1 g/mol/276.2 g/mol = 0.4935. The molecular formula is obtained by multiplying the empirical formula by this factor: \(K_2H_2P_2O_8 * 0.4935 = K_2HPO_4\). Therefore, the molecular formula of the compound is \(K_2HPO_4\).The molecular formula of the given compound having a composition of 28.73% K, 1.48% H, 22.76% P, and 47.03% O with a molar mass of 136.1 g/mol is \(K_2HPO_4\). The empirical formula of the compound is \(K_2H_2P_2O_8\). The compound's molecular formula is calculated by determining the factor by which the empirical formula should be multiplied to obtain the molecular formula. The factor is M(molecular formula)/M(empirical formula) = 136.1 g/mol/276.2 g/mol = 0.4935. The molecular formula of the compound is obtained by multiplying the empirical formula by this factor, resulting in the molecular formula \(K_2HPO_4\).For more questions on empirical formula
https://brainly.com/question/13058832
#SPJ8
The correct question would be as
The composition of a compound is 28.73% K. 1.48% H, 22.76% P, and 47.03% O. The molar mass of the compound is 136.1 g/mol. What is the Molecular Formula of the compound?
\(KH_2PO_4\\KH_3PO_4\\K_2H_4P_20_{12}\\K_2H_3PO_6\)
draw the major thermodynamic and kinetic products of the reaction. draw the kinetic product. select draw rings more erase select draw rings more erase select draw rings more erase c h br draw the thermodynamic product.
Answers
The major thermodynamic product of the reaction between C₅H₈ and HBr is the most stable product, which is usually the one with the lowest free energy. On the other hand, the major kinetic product is the product that is formed through a faster and less controlled reaction pathway.
This thermodynamic product product is formed through a slower and more controlled process, which allows for the formation of stronger chemical bonds and results in a more stable final product.
This kinetic product is formed due to its lower activation energy, which makes it easier to form, but it may not necessarily be the most stable product.
In the case of C₅H₈ and HBr, the reaction can result in both thermodynamically and kinetically stable products, meaning that there can be multiple products formed, each with different stability properties. The thermodynamically stable product is the one with the lowest free energy, while the kinetically stable product is the one with the lower activation energy.
In summary, the major thermodynamic product of the reaction is the most stable product, while the major kinetic product is the product that is formed through a faster and less controlled reaction pathway. However, it is important to note that the exact products of the reaction can vary based on the reaction conditions and reactant concentrations.
To know more about kinetic products click on below link:
https://brainly.com/question/22616533#
#SPJ11
Complete question:
What are themajor thermodynamic and kinetic products of the reaction.
C₅H₈ + HBr (1 equivalent) ------> thermodynamically stable + Kinetically stable
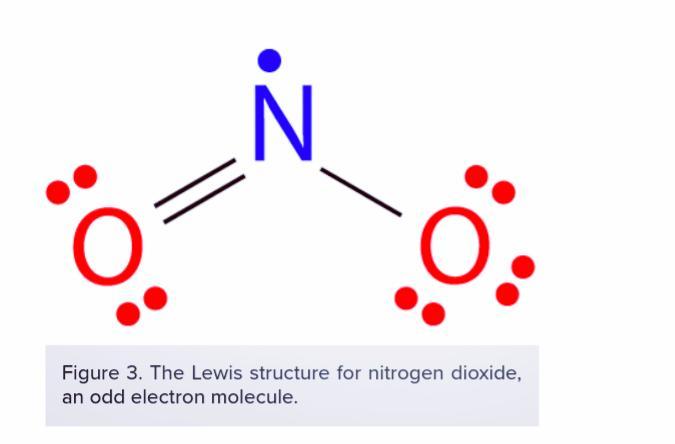
Octet Rule states that atoms of main-group elements tend to combine in such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas. Depending on this fact answer the following question :
*What is an incomplete octet?
*What is an odd-electron molecule?
*Why are there extra electrons in the expanded octet?
Answers
Second question: There are a number of molecules whose total number of valence electrons is an odd number. It is not possible for all of the atoms in such a molecule to satisfy the octet rule. An example is nitrogen dioxide (NO2). Each oxygen atom contributes six valence electrons and the nitrogen atom contributes five for a total of seventeen.
Third question: Expanded octet occurs when an atom is able to have more than 8 valence electrons. For example, in SO₃, the sulfur atom forms 6 covalent bonds, hence it has 12 valence electrons. Expansion of octet is possible only from Period 3 elements onwards, due to the presence of low-lying empty d orbitals that can accommodate the extra electrons.
Can you mark me as brainliest?
Answer:
Incomplete Octet
In some compounds, the number of electrons surrounding the central atom in a stable molecule is fewer than eight. Beryllium is an alkaline earth metal and so may be expected to form ionic bonds. However, its very small size and somewhat higher ionization energy compared to other metals actually lead to beryllium forming primarily molecular compounds. Since beryllium only has two valence electrons, it does not typically attain an octet through sharing of electrons. The Lewis structure of gaseous beryllium hydride (BeH 2 ) consists of two single covalent bonds between Be and H
Odd-Electron Molecules
There are a number of molecules whose total number of valence electrons is an odd number. It is not possible for all of the atoms in such a molecule to satisfy the octet rule. An example is nitrogen dioxide (NO 2 ). Each oxygen atom contributes six valence electrons and the nitrogen atom contributes five for a total of seventeen.
Expanded Octets
Atoms of the second period cannot have more than eight valence electrons around the central atom. However, atoms of the third period and beyond are capable of exceeding the octet rule by having more than eight electrons around the central atom. Starting with the third period, the d sublevel becomes available, so it is possible to use these orbitals in bonding, resulting in an expanded octet.
Phosphorus and sulfur are two elements that react with halogen elements and make stable compounds with expanded octets. In phosphorus pentachloride, the central phosphorus atom makes five single bonds to chlorine atoms and as a result has ten electrons surrounding it
Explanation:


which phase has the highest entropy?
a. gas
b. liquid
c. solid
d. aqueous
Answers
Answer:
Gas>Liquid>Solid
Explanation:
Entropy by definition is the degree of randomness in a system. If we look at the three states of matter: Solid, Liquid and Gas, we can see that the gas particles move freely and therefore, the degree of randomness is the highest.
As the reaction goes through phases, the phase that has the highest entropy is the gas state. This is further explained below. Option A is correct.
What is entropy?A thermodynamic number that represents the inability of a system's thermal energy to be converted into mechanical work, and is commonly understood as the system's degree of disorder.
In conclusion, we have that as the reaction goes through phases, the gaseous state has the highest entropy.
Read more about Chemical reaction
https://brainly.com/question/16416932
Choose the compound that exhibits hydrogen bonding as its strongest intermolecular force. Choose the compound that exhibits hydrogen bonding as its strongest intermolecular force. CH2Br2 CH3NH2 LiF C3H8 CF4
Answers
Answer:
The given molecules are:
CH2Br2
CH3NH2
LiF
C3H8
CF4.
Which compound consists of the hydrogen bond as the strongest intermolecular force.
Explanation:
The hydrogen bond is the electrostatic force of attraction that exists between the covalently bonded H-atom of one molecule and a high electronegative atom (N, O, F) of another molecule.
For example, H-bonding in water is represented below:
Among the given molecules,
CH2Br2 does not have H-bond because there is no either N or O or F atom in it.
In LiF also there is no H-atom and no hydrogen bond.
C3H8 also does not have H-bond in it.
CF4 also does not have H-atom or hydrogen bond in it.
The answer is CH3NH2(methylamine).
It has an intermolecular hydrogen bond in it as shown in the attachment.
The dashed line represents the H-bond.


Lewis structure AgBr2
Answers
Answer:
Br-Ag-Br
Explanation:

What is the amount (in mole) of sodium trioxocarbonate (IV) in 5.3g of the compound? (Na2CO3 = 106)
Answers
Answer:
To calculate the amount (in mole) of sodium trioxocarbonate (IV) in 5.3g of the compound , you can use the formula:
Amount (in moles) = Mass of substance / Molecular mass
The molecular mass of sodium trioxocarbonate (IV) (Na2CO3) is 106 g/mol, as given in the question. Substituting the values in the formula, we get:
Amount (in moles) = 5.3 g / 106 g/mol = 0.05 mol
Therefore, the amount (in mole) of sodium trioxocarbonate (IV) in 5.3g of the compound is 0.05 mol.
Note that the given molecular mass of Na2CO3 must be used to obtain the correct answe12 .
Explanation:
Please help! Virtual Chemical vs Physical changes

Answers
Answer:
Sometimes, it can be difficult to tell if a chemical or physical change is taking place. In the video, Dr. Jeff and the team explore a few different reactions to determine if they are chemical or physical changes, by figuring out if the material made after the reaction was present before the reaction. Chopping a banana.
Explanation:
Part B Identify the sets of quantum numbers that describe all the electrons in the ground state of a neutral beryllium atom, Be. Each set is ordered (n, l, me, ms). Drag the appropriate items to their respective bins. View Available Hint(s) Reset Help 2,0,0,1/2 2,1,-1,1/2 2,1,0,1/2 1,0,0,-1/2 2,1,-1,-12 1,0,0,1/2 2,1,0,-1/2 2,0,0,-1/2 Electrons in Be Electrons not in Be Submit
Answers
Answer: The set of quantum numbers for the electrons in Be atom are (2, 1, -1, 1/2), (1, 0, 0, 1/2), (1, 0, 0, -1/2) and (2, 0, 0, -1/2)
Explanation:
There are 4 quantum numbers:
Principal Quantum number (n) specifies the energy of the electron in a shell.Azimuthal Quantum number (l) specifies the shape of an orbital. The value of it lies in the range of 0 to (n-1)Magnetic Quantum number (m) specifies the orientation of the orbital in space. The value of it lies in the range of -l to +lSpin Quantum number (s) specifies the spin of an electron in an orbital. It can either have a value of \(+\frac{1}{2}\) or \(-\frac{1}{2}\)Berylium (Be) is the 4th element of periodic table having electronic configuration of \(1s^22s^2\)
For electrons in 1s-orbital, the quantum numbers can be:For first electron:
\(n=1\\l=0\text{ (for s-subshell)}\\m=0\\s=+\frac{1}{2}\)
For second electron:
\(n=1\\l=0\\m=0\\s=-\frac{1}{2}\)
For electrons in 2s-orbital, the quantum numbers can be:For first electron:
\(n=2\\l=0\text{ (for s-subshell)}\\m=0\\s=+\frac{1}{2}\)
For second electron:
\(n=2\\l=0\text{ (for s-subshell)}\\m=0\\s=-\frac{1}{2}\)
Hence, the set of quantum numbers for the electrons in Be atom are (2, 1, -1, 1/2), (1, 0, 0, 1/2), (1, 0, 0, -1/2) and (2, 0, 0, -1/2)
What is true about radioactive isotopes of an atom?
They are more abundant.
They are more stable.
They are less stable.
They are more scarce.
Answers
Answer: They are less stable
Explanation:
Answer:
The answer is C) They are less stable
Explanation:
Different isotopes of the same element have the same number of protons in their atomic nuclei but differing numbers of neutrons. Radioisotopes are radioactive isotopes of an element. They can also be defined as atoms that contain an unstable combination of neutrons and protons, or excess energy in their nucleus.
:)
the increase in atomic radius as you go down the periodic table is caused by?
Answers
Answer: The number of energy levels increases as you move down a group as the number of electrons increases. Each subsequent energy level is further from the nucleus than the last. Therefore, the atomic radius increases as the group and energy levels increase.
A sample of pure copper has a volume of 3.75 cm3. Calculate its mass
Answers
Answer:
33.60 g
Explanation:
In order to solve this problem we need to know the density of pure copper. That value can be found on the periodic table or in textbooks: 8.96 g/cm³.
Knowing the density of copper, we can calculate the mass of a sample that occupies 3.75 cm³:
Density = mass / volumeMass = Density * volume8.96 g/cm³ * 3.75 cm³ = 33.60 gConvert the following temperature to kelvin:a) 113°C, the melting point of sulfur,b) 37°C, the normal body temperature,c) 357°C, the boiling point mercury.
Answers
The temperature to kelvin at 113°C is 386.15 K, at 37°C is 310.15 K and at 357°C is 630.15 K.
Equation :To find Kelvin use formula,
Kelvin = 273.15 + degree Celsius
So,
(a) The melting point of sulfur at 113 degree Celsius
273.15 + 113 = 386.15 K
(b) The normal body temperature at 37 degree Celsius
273.15 + 37 = 310.15 K
(c) The boiling point of mercury at 357 degree Celsius
273.15 + 357 = 630.15 K
What is temperature?Temperature is a measure of hotness and coldness that can be expressed on a variety of scales, including Fahrenheit and Celsius. Temperature indicates the direction in which heat energy will flow from a hot body to a colder body to a lower temperature.
The total energy of the molecular motion inside the object is represented by the heat open object. Temperature is a measure of the thermal energy or average heat of a substance's molecules.
To know more about motion visit :
https://brainly.com/question/22810476
#SPJ9
How could you distinguish a compound from a mixture
Answers
Answer:
Compound are substances which can be formed by chemically combining two or more elements. Mixtures are substances that are formed by physically mixing two or more substances.3. Does entropy increase or decrease in the following processes?
A. Complex carbohydrates are metabolized by the body, converted into simple sugars.
Answer: Increase
es-lesund
B. Steam condenses on a glass surface.
Answer:
decreare
-->
MgCl2(s)
C. Mg(s) + Cl2(g)
correct
Answer:
Answers
Answer:
hope it helps much as you can

The molar mass of C3H8O2 is.
(A) 60.09 g/mole
B) 29.02 g/mole
69.02 g/mole
76.09 g/mole
52.01 g/mole
E
Answers
Answer:
76.09 g/mole
Explanation:
The atomic mass of carbon is 12.011 g/mol.The atomic mass of hydrogen is 1.00794 g/mol.The atomic mass of oxygen is 15.9994 g/mol.So, the molar mass is 3(12.011)+8(1.00794)+2(15.9994), which is about 76.09 g/mole
1b. Suppose that you were titrating a 100 mL acid solution with the 0.1 M NaOH solution that you made. You performed the titration multiple times and obtained the data below. Complete the data table below. Show work on a separate piece of paper/ the back of this paper.
Step 1: Write and balance the chemical equation (only need to do this once for each titration)
Step 2: Use the molarity and mL of base used to find the moles of base it took to neutralize the acid
Step 3: Calculate moles of acid neutralized
Step 4: Calculate molarity of acid
Step 5: Calculate pH
1c. Calculate the most likely pH of the acid solution by finding the average of all the pH's you found in each of your multiple titrations. We find the average to minimize human errors made while titrating.

Answers
The moles of NaOH used is 0.0008 moles
The molarity of the acid is 0.008 M
What is the molarity of the acid?The molarity of the acid is found as follows:
Moles of NaOH used = concentration of NaOH × volume of NaOH used
the average volume of NaOH used = 8.0 mL
moles of NaOH = 0.1 M × 8.0 mL
moles of NaOH = 0.0008 moles
Molarity of acid:
Assuming the acid is monobasic, the mole ratio of acid to base is 1 : 1
The volume of acid used is 100 mL
The molarity of acid = moles of acid / volume of acid in liters
The molarity of acid = 0.0008 moles / 0.1 L
The molarity of acid = 0.008 M
Learn more about molarity at: https://brainly.com/question/30404105
#SPJ1
Determine whether each of the following salts will form a solution that is acidic, basic, or pH-neutral.
a. KCl
b. NaClO
Answers
Answer:
a. Neutral
b. Basic
Explanation:
To determine which of the salts are acidic, neutral or basci we should dissociate them and determine if the ions, can make hydrolysis to water.
KCl → K⁺ + Cl⁻
We need to know, where do the ions come from. In this case, K⁺ comes from the KOH which is a strong base and Cl⁻ comes from the HCl, a strong acid. In conclussion, both are the conjugate weak acid and base, respectively. They do not make hydrolysis, so this salt is neutral. No protons or hydroxides are given.
NaClO → Na⁺ + ClO⁻
The Na⁺ comes from the NaOH, it is the conjugate weak acid from a strong base, while the ClO⁻ comes from the HClO, a weak acid. This means that the ClO⁻ can react to water, to make hydrolysis. The equilibrium will be:
ClO⁻ + H₂O ⇄ HClO + OH⁻
We are giving hydroxides to medium, so the salt is basic.
THEORY 1. illustrate the formation of the Compound AIC 13 Electron dot representation.
Answers
The electron representation shows the electrons in the atoms as dots as in the image attached.
What is electron dot representation?An electron dot representation, also known as a Lewis dot structure or electron dot diagram, is a way of representing the valence electrons of an atom using dots around the symbol of the element.
Valence electrons are the outermost electrons of an atom, and they play an important role in chemical bonding. The electron dot representation shows the valence electrons as dots around the symbol of the element, with each dot representing one valence electron.
Learn more about electron dot:https://brainly.com/question/25929171
#SPJ1

What was life like in the United States during the 1930s?
Answers
The 1930s saw natural disasters as well as manmade ones: For most of the decade, people in the Plains states suffered through the worst drought in American history, as well as hundreds of severe dust storms, or "black blizzards," that carried away the soil and made it all but impossible to plant crops.
PLEASE NEEEED HELP drag each label to the correct location on the image here’s one way to follow the scientific method. Place the missing steps in the correct position in the process

Answers
Answer: Make an observation -> Ask a question. -> Construct a hypothesis. -> Test the hypothesis with an investigation -> Analyze the data -> Explain the results -> (Left) The hypothesis is true ------ (Right) The hypothesis is false. -> Communicate the results.
Explanation:
Make an observation -> Ask a question. -> Construct a hypothesis. -> Test the hypothesis with an investigation -> Analyze the data -> Explain the results -> (Left) The hypothesis is true ------ (Right) The hypothesis is false. -> Communicate the results.
Answer:
Make an observation -> Ask a question. -> Construct a hypothesis. -> Test the hypothesis with an investigation -> Analyze the data -> Explain the results -> (Left) The hypothesis is true ------ (Right) The hypothesis is false. -> Communicate the results.
Explanation:
The molar heat capacity of copper is adequately given by the equation Cp = 4.7310-5 * T3 (J/molK). What is the absolute entropy of copper at 20K?
a.) 2.410-6 J/molK
b.) 0.019 J/mol*K
c.) 0.13 J/mol*K
d.) 1.9 J/mol*K
Answers
Explanation:
calculate the change in velocity of an eagle that accelerates downward at 4.6m/s for a time of 5second?
The molar heat capacity of copper is adequately given by the equation Cp = 4.7310-5 * T3 (J / molK ). 1.9 J / mol * K is the absolute entropy of copper at 20K.
What is molar heat capacity ?
The amount of energy needed to add one mole of a chemical substance in the form of heat in order to raise its temperature by one unit is known as the material's molar heat capacity.
The molar heat capacity is the heat capacity for one mole of a material, whereas the specific heat capacity, which is sometimes just termed specific heat, is the heat capacity for one gram of a substance.
The molar heat capacity ( Cp ) of a substance as a function of temperature, and then plot the value of Cp / T versus temperature. The absolute entropy of a material at any temperature T is represented as the area under the curve between 0 K and T.
Thus, the option D is correct.
To learn more about molar heat capacity follow the link below;
https://brainly.com/question/6363778
#SPJ12
What happens to the pH when a a small amount of acid is added to a buffered solution?
A.the pH goes up to 14.
B.The pH goes down to 1.
C.The pH stays about the same.
D.The pH goes to 7.
Answers
C. The pH stays about the same.
A buffered solution resists changes in pH upon addition of small amounts of acid or base. The buffer system in the solution will react with the added acid, keeping the pH relatively constantAnswer:
C.The pH stays about the same.
Explanation:
Buffer reactions maintain stable pH of solutions.
What is paper made of?
Answers
Paper used as a writing material is made of pulp (wood).
What is paper?Paper is a sheet material used for writing on or printing on (or as a non-waterproof container), usually made by draining cellulose fibres from a suspension in water.
Paper is made from cellulose found in trees, which are the main source of cellulose fibre (or woodpulp). Besides woodpulp, paper can be made from other materials such as cotton, flax, esparto, straw, hemp, manilla and jute.
Wood pulp is usually a softwood, used for pulping to make paper.
Learn more about pulp at: https://brainly.com/question/23590026
#SPJ1