In an electrolytic cell, the cathode is where _____ A) anions are attracted to B) a graphite electrode is used C) oxidation occurs D) reduction occurs E) electrons are created
Answers
In an electrolytic cell, the cathode is where (D) reduction occurs.
Reduction is the gain of electrons, and the cathode is the electrode where electrons are supplied to the system. The cathode is connected to the negative terminal of the power supply and is thus negatively charged. When the positively charged cations in the electrolyte solution migrate towards the cathode, they gain electrons and are reduced.
The reduction reaction at the cathode is the half-reaction that consumes electrons, and it is the opposite reaction to the oxidation reaction that occurs at the anode.
Therefore, option D ("reduction occurs") is the correct answer. Option A is incorrect because anions are attracted to the anode, which is connected to the positive terminal of the power supply, and option E is incorrect because electrons are not created but rather supplied from the external power source. Option B is irrelevant to the function of the cathode in an electrolytic cell.
To know more about the electrolytic cell refer here :
https://brainly.com/question/861659#
#SPJ11
Related Questions
How did mitochondria
become part of the cell?
Answers
Answer: The mitochondria, as bacteria, only wanted to rob the host cells of their energy and then leave them to die. But the bacteria soon realized the benefit of working together with simple cells. The simple cells provide them with antioxidants to protect them from free radicals and toxic reactive oxygen species that the mitochondria generate as a byproduct of energy production.
Hope dis helps!
:)
In the Haber Process, ammonia is synthesized from nitrogen andhydrogen:
N2 (g) + 3H2 -----> 2NH3(g)
ΔG at 298K for this reaction is -33.3 kj/mol. the valuef ΔG at 298 K for a reaction mixture that consists of 1.9 atmN2, 1.6 atm H2 and 0.65 atm NH3 is________.
a.) -3.86 x 103
b.) -1.8
c.) -7.25 x 103
d.) -40.5
e.) -104.5
Answers
The value of ΔG at 298 K for a reaction mixture containing 1.9 atm N2, 1.6 atm H2, and 0.65 atm, the answer is (a) -3.86 × 10^3.
NH3 can be calculated using the equation:
ΔG = ΔG° + RT ln(Q)
where ΔG is the standard Gibbs free energy change, ΔG° is the standard Gibbs free energy change at standard conditions, R is the gas constant, T is the temperature in Kelvin, and Q is the reaction quotient.
In this case, we are given ΔG° as -33.3 kJ/mol. To calculate Q, we need to use the partial pressures of the gases in the reaction mixture. The reaction stoichiometry tells us that the ratio of the partial pressures of N2, H2, and NH3 is 1:3:2. Therefore, we can write:
Q = (P(NH3))^2 / (P(N2) * P(H2)^3)
Plugging in the given values of P(N2) = 1.9 atm, P(H2) = 1.6 atm, and P(NH3) = 0.65 atm, we can calculate Q. Then, using the value of R = 8.314 J/(mol·K) and the temperature T = 298 K, we can substitute these values into the equation and solve for ΔG.
The calculated value of ΔG at 298 K for the given reaction mixture is approximately -3.86 × 10^3 J/mol. This value is equivalent to -3.86 kJ/mol. Therefore, the answer is (a) -3.86 × 10^3.
To learn more about Haber Process here brainly.com/question/30928282
#SPJ11
Following Rutherford, Niels Bohr developed an improved model of the atom in which the electrons moved around the nucleus in quantized energy shells. Which of the following phenomena could Bohr's model explain but Rutherford's model could not?
Group of answer choices:
spectral emission lines from hydrogen atoms
wave-particle duality of electrons
protons accounting for roughly half of an atom's mass
alpha particles passing through gold foil undeterred
Answers
Answer:
spectral emission lines from hydrogen atoms
Explanation:
Please help me what is the answer

Answers
There are 20.8 moles of propanol
Further explanationThe mole is the number of particles(molecules, atoms, ions) contained in a substance
1 mol = 6.02.10²³ particles
Can be formulated
N=n x No
N = number of particles
n = mol
No = Avogadro's = 6.02.10²³
1.2 x 10⁵ molecules of propanol
\(\tt \dfrac{1.25\times 10^{25}}{6.02\times 10^{23}}=20.8~moles\)
How would pressure change if i. the area is doubled by keeping the force constant ii. the force is doubled by keeping the area constant.
Help!!
-From Nepal
Answers
Answer:
i. If the area is doubled keeping the force constant, then pressure will be halved.
ii. If force is doubled keeping area constant, then pressure will also be doubled.
I hope that my answer helped you!!
-From Nepal
What is the name of Pb(NO3)2? Explain how you determined the bond type and the steps you used to determine the naming convention for the compound.
Answers
This chemical is known as lead (II) nitrate. It is an ionic assembly (salt compound) comprised of lead cations in the +2 oxidation state. With regard to the naming convention, each lead (II) cation is paired with two nitrate anions, each having a charge of -1.
What is a naming convention in Chemistry?Chemical nomenclature is a set of principles for naming chemical substances in a systematic manner. The International Union of Pure and Applied Chemistry designed and developed the most widely used nomenclature in the world (IUPAC).
The basic goal of chemical nomenclature is to guarantee that no ambiguity exists between a spoken or written chemical name and the chemical compound to which the name refers. Each chemical name should only relate to one substance.
It is required to indicate the charge of these cations or compounds containing these cations when identifying them. Ionic compounds are formed when cations and anions interact. The cation of an ionic compound is named first, followed by the anion. When writing their chemical formulae, they use the same format.
Learn more about naming conventions:
https://brainly.com/question/14326884
#SPJ1
The process of moving water from its source to places where humans use it is called water diversion. true or false
Answers
Answer:
That is def true
Explanation:
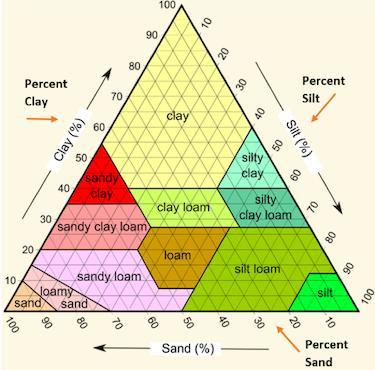
Jonathan used the following soil triangle to identify a sample of soil as sandy clay. Which description of soil likely allowed Jonathan to make this identification.
A. Mostly large grains, with a sticky texture, 55% sand, 40% clay, 5% silt.
B. Mostly large grains, with a gritty texture, 45% sand, 5% clay, 45% silt.
C. Mostly small grains, with a smooth texture, 30% sand, 5% clay, 65% silt.
D. Mostly small grains, with a sticky texture, 30% sand, 50% clay, 20% silt.
Answers
Answer:
Mostly large grains, with a sticky texture, 55% sand, 40% clay, and 5% silt
Explanation: I took the test, hope it helps.
Mostly large grains, with a sticky texture, 55% sand, 40% clay, 5% silt. The correct option is A.
What is soil triangle?One side of the triangle represents sand, the other side represents clay, and the third side represents silt.
A soil texture triangle is used to classify a soil's texture class. The percentages of sand, silt, and clay are scaled on the sides of the soil texture triangle.
Clay percentages are read across the triangle from left to right. Silt is read from top to bottom, upper right to bottom.
Jonathan used the soil triangle to identify a sample of soil as sandy clay; the soil description that allowed Jonathan to make this identification is 55% sand, 40% clay, and 5% silt, with mostly large grains and a sticky texture.
Thus, the correct option is A.
For more details regarding soil triangle, visit:
https://brainly.com/question/1698808
#SPJ2
How many joules are needed to change the temperature of 40 g of water from 33 0C to 23 0C?
Answers
The properties of several unknown solids were measured. Solid Melting Point Other Properties A 1000 "C does not conduct electricity B 850 C conducts electricity in the liquid state, but not in the solid state c | 750 ℃ conducts electricity in the solid state D 150 C does not conduct electricity Classify these solids. Lonic Molecular Metallic Covalent also known as covalent network solids, or macromolecular solids
Answers
Answer: A -Covalent Solid, B - Ionic Solid , C - Metallic Solid , D -
Molecular solid
Explanation: Covalent Solid:- Atoms are held together by covalent bond.
Ionic Solid:-A solid compound formed by chemical reaction of a cation(+) and with an anion (-) . Metallic Solid:-Atoms of metal held together by metallic bonds. Molecular solid:- molecules are held together by Vander waal forces. A >1000 °C does not conduct electricity.B 850 °C conducts electricity in the liquid state.C 750 °C conducts electricity in the solid state.D 150 °C does not conduct electricity.
FIND MORE:- Brainly.in
#SPJ4
HELP ASAP!!
A student mixes baking soda and vinegar in a glass. The results are shown at left. Do you think any new substances are being created in this mixture? If so, how do you know?
Answers
Hope this helps pls mark me brainliest
A gold bar contains 5 moles of gold. How many atoms of gold are present in the bar?
Answers
Answer:
= 3.01 x 10²³ atoms of gold (Au)
Explanation:
1 mole of any substance => 1 Avogadro's Number of particles = 6.02 x 10²³ particles.
∴ for 5 moles of gold atoms => 5 moles x 6.02 x 10²³ atoms/mole
= 3.01 x 10²³ atoms of gold (Au)
What is the pH of a 2.20 M solution of the weak acid CH3CO2H, given that the Ka of the acid is 1.76×10−5? The equilibrium expression is:
CH3CO2H(aq)+H2O(l)⇋H3O+(aq)+CH3CO−2(aq)
Answers
The pH of the 2.20 M solution of the weak acid CH3CO2H can be calculated using the equilibrium expression and the dissociation constant (Ka) of the acid.
What is the pH of the solution?To determine the pH of the solution, we need to consider the dissociation of the weak acid CH3CO2H. The equilibrium expression shows the formation of H3O+ ions (hydronium ions) and CH3CO−2 ions (acetate ions) from the dissociation of CH3CO2H in water.
The Ka value represents the acid dissociation constant and is given as 1.76×10−5. This value indicates the degree of dissociation of the acid. Since the acid is weak, it only partially dissociates, and we can assume that the initial concentration of CH3CO2H remains relatively unchanged.
To find the pH, we need to determine the concentration of H3O+ ions. Since CH3CO2H is a weak acid, we can approximate the concentration of H3O+ ions to be equal to the concentration of the acid that dissociates. Therefore, the concentration of H3O+ ions is approximately 2.20 M.
Using the equation pH = -log[H3O+], we can calculate the pH of the solution.
In this case,
\(pH = -log(2.20) = -log(2.20) = 0.657.\)
Therefore, the pH of the 2.20 M solution of CH3CO2H is approximately 0.657.
Learn more about pH
brainly.com/question/32445629
#SPJ11
35. a
When aqueous iron (III) chiondes added to aqueous potassium iodide a chemical con
ours and lodine is formed
Which statement is correct?
A todide sons are oxidised, they gain electrons in this reaction
lodide ions are oxidised, they lone electrons in this reaction
C trond) chionde is oxidised in this reaction
D Neither iodide ions nor iron (III) chlonde is ondised in this reachon

Answers
8.0 mol AgNO3 reacts with 5.0 mol Zn in
a single replacement reaction.
2AgNO3 + Zn → 2Ag + Zn(NO3)2
How many moles of Ag form from 8.0
mol AgNO3?
[?] mol Ag
Round your answer to the tenths place.

Answers
Taking into account the reaction stoichiometry, 8 moles of Ag can be produced from 8 moles of AgNO₃ and 5 moles of Zn.
Reaction stoichiometryIn first place, the balanced reaction is:
2 AgNO₃ + Zn → 2 Ag + Zn(NO₃)₂
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
AgNO₃: 2 moles Zn: 1 mole Ag: 2 moles Zn(NO₃)₂: 1 moleLimiting reagentThe limiting reagent is one that is consumed first in its entirety, determining the amount of product in the reaction. When the limiting reagent is finished, the chemical reaction will stop.
Limiting reagent in this caseTo determine the limiting reagent, it is possible to use a simple rule of three as follows: if by stoichiometry 1 mole of Zn reacts with 2 moles of AgNO₃, 5 moles of Zn reacts with how many moles of AgNO₃?
\(amount of moles of AgNO_{3}= \frac{5 moles of Znx2 moles of AgNO_{3}}{1 mole of Zn}\)
amount of moles of AgNO₃= 10 moles
But 10 moles of AgNO₃ are not available, 8 moles are available. Since you have less moles than you need to react with 5 moles of Zn, AgNO₃ will be the limiting reagent.
Moles of Ag formedConsidering the limiting reagent, the following rule of three can be applied: if by reaction stoichiometry 2 moles of AgNO₃ form 2 moles of Ag, 8 moles of AgNO₃ form how many moles of Ag?
\(amount of moles of Ag=\frac{8 moles of AgNO_{3}x2 moles of Ag }{2 moles of AgNO_{3}}\)
amount of moles of Ag= 8 moles
Then, 8 moles of Ag can be produced from 8 moles of AgNO₃ and 5 moles of Zn.
Learn more about the reaction stoichiometry:
brainly.com/question/24741074
brainly.com/question/24653699
#SPJ1
The element with 5 electrons in its 3d sublevel is...?
Answers
Answer:
manganese, Mn
Explanation:
From the question, we have;
The number of electrons in the 3d sub level = 5
The kind of element = d-block element
On the periodic table, the elements with electrons in the 3d-sublevel in increasing order are;
Sc, 3d¹ 4s²
Ti 3d² 4s²
V 3d³ 4s²
Cr 3d⁵ 4s¹
Mn 3d⁵ 4s²
Fe 3d⁶ 4s²
Co 3d⁷ 4s²
Ni 3d⁸ 4s²
Cu 3d¹⁰ 4s¹
Zn 3d¹⁰ 4s²
The elements with 5 electrons in their 3d-sublevel are chromium Cr and manganese, Mn however the electronic configuration of chromium is given as 3d⁵ 4s¹ rather than 3d⁴ 4s² due to the minor difference in the gap of energy between the 3d and the 4s orbital as such the element that has the main characteristic of 5 electrons in its 3d sub level is manganese, Mn.
What property of water accounts for its ability to insulate areas around it?
Answers
Answer:
Floating ice can insulate bodies of water. Answer: b. Discuss how a high specific heat helps to buffer temperature for organisms. Water's high specific heat is important to life because...
Explanation:
Consider the word equation. Calcium hydroxide hydrochloric acid mc012-1. Jpg calcium chloride water Which is the corresponding formula equation? Upper C a upper C l subscript 2 right arrow upper C a (s) plus upper C l subscript 2 (g). Upper C a upper O (s) plus upper H subscript 2 upper O (l) right arrow upper C a (upper O upper H) subscript 2 (a q). Upper N a upper O upper H (a q) plus 2 upper H upper C l (a q) right arrow upper N a upper C l (a q) plus 2 upper H subscript 2 upper O (l). Upper C a (upper O H) subscript 2 (s) plus 2 upper H upper C l (l) right arrow upper C a upper C l subscript 2 (a q) plus 2 upper H subscript 2 upper O (l).
Answers
The corresponding formula equation is:
Upper Ca (upper OH) subscript 2 (s) plus 2 upper H upper Cl (l) right arrow upper Ca upper Cl subscript 2 (aq) plus 2 upper H subscript 2 upper O (l)
To obtain the word equation, we shall write out the balanced molecular equation. This is given below:
Calcium hydroxide => Ca(OH)₂
Hydrochloric => HCl
Calcium chloride => CaCl₂
Water => H₂O
Calcium hydroxide + Hydrochloric —> Calcium chloride + water
Ca(OH)₂ + HCl —> CaCl₂ + H₂O
There are 2 atoms of Cl on the right side and 1 atom on the left. It can be balance by writing 2 before HCl as shown below:
Ca(OH)₂ + 2HCl —> CaCl₂ + H₂O
There are a total of 4 atoms of H on the left side and 2 atoms on the right side. It can be balance by writing 2 before H₂O as shown below:
Ca(OH)₂ + 2HCl —> CaCl₂ + 2H₂O
Now, the equation is balanced.
Thus, the corresponding word equation is:
Upper Ca (upper OH) subscript 2 (s) plus 2 upper H upper Cl (l) right arrow upper Ca upper Cl subscript 2 (aq) plus 2 upper H subscript 2 upper O (l)
Learn more about balancing equation: https://brainly.com/question/15538713
Answer:
Upper Ca (upper OH) subscript 2 (s) plus 2 upper H upper Cl (l) right arrow upper Ca upper Cl subscript 2 (aq) plus 2 upper H subscript 2 upper O (l)
list Two examples of seeds that are dispersal by water
Answers
Answer:
okay
Explanation:
Coconut, palm, mangroves, water lily, water mint, are a few examples of plants whose seed are dispersed by the water.
which safety concerns should one pay attention to when using 1-indanone?
a. do not mix with acids
b. None of the answer correct
c. keep it at a temperature 0 degree celcius
d. keep it in an inert environment
Answers
Option C is the correct answer about the safety precautions during use of 1-Indanone i.e. none of the above option is correct.
-Indanone is a chemical compound with the formula C6H4(CH2)2CO. It's one of two isomeric benzocyclopentanones, along with 2-indanone. It is an uncolored solid. The enzyme indanol dehydrogenase uses 1-indanone as a substrate.
11H-Indeno-[1,2-b] can be synthesised using 1-Indanone.
-quinolin-10-ylamine, a potential acetylcholinesterase inhibitor for Alzheimer's disease treatment.
When using 1-Indanone, keep the following safety precautions in mind:- Wash thoroughly after handling. Wash contaminated clothing before reusing it. Use with proper ventilation. Avoid coming into contact with your eyes, skin, or clothing. Keep the container tightly shut. Ingestion and inhalation should be avoided.
Store away from incompatible substances in a cool, dry, well-ventilated area. Containers should be tightly closed.
To know more about Chemical safety precautions go through:-
https://brainly.com/question/29830852
#SPJ4
Two intermetallic compounds, A3B and AB3, ex- ist for elements A and B. If the compositions for A3B and AB3 are 91. 0 wt% A–9. 0 wt% B and 53. 0 wt% A–47. 0 wt% B, respectively, and element A is zirconium, identify element B
Answers
The compositions of the intermetallic compounds A3B and AB3 are given as percentages of element A and element B, respectively.
Another approach to identifying element B would be to perform chemical analysis on small samples of the compounds. This would involve determining the elemental composition of the samples using techniques such as X-ray fluorescence (XRF) or inductively coupled plasma mass spectrometry (ICP-MS). From the elemental composition, element B could be identified based on its position in the periodic table and its known properties. However, the compositions do not provide enough information to determine the identity of element B.
In order to identify element B, additional information is needed. One possible way to identify element B is to compare the known properties of the compounds formed by A and B. For example, if A forms a cubic structure and B forms a tetragonal structure, then element B is likely zirconium (Zr). However, without additional information, it is not possible to definitively identify element B using only the given compositions of A3B and AB3.
Learn more about compounds visit: brainly.com/question/29547278
#SPJ4
Fusion of hydrogen releases energy because O Fusion breaks the electromagnetic bonds between hydrogen atoms, releasing energetic photons. The mass of a helium nucleus is smaller than the mass of four protons The mass of a helium nucleus is larger than the mass of four protons The size of a proton is larger than the size of a helium nucleus None of the above is true. 20 Fusion in the core of a stable massive star cannot proceed beyond iron because It would require temperatures that even stars cannot generatel The fusion of iron nuclei is impossible under any circumstances. Iron nuclei are on top of the binding energy curve so iron fusion does not release energy. It is so massive that a black hole must result 000
Answers
Question 19: The fusion of hydrogen releases energy because the mass of a helium nucleus is smaller than the mass of four protons.Question 20: Fusion in the core of a stable massive star cannot proceed beyond iron because it would require temperatures that even stars cannot generate.
Question 19 addresses the reason why the fusion of hydrogen releases energy. The correct statement is that the mass of a helium nucleus is smaller than the mass of four protons. This mass difference results in the release of energy during fusion reactions. In fusion, hydrogen nuclei (protons) combine to form helium nuclei, and in the process, some mass is converted into energy according to Einstein's famous equation, E=mc^2. This energy is released in the form of photons, which can be observed as light and heat.
Question 20 explains why fusion in the core of a stable massive star cannot proceed beyond iron. The correct statement is that it would require temperatures that even stars cannot generate. Fusion reactions in stars involve the fusion of lighter elements to form heavier elements, releasing energy in the process.
However, fusion reactions that produce elements heavier than iron require extremely high temperatures and pressures, which are not achievable in the core of a stable massive star. Iron has the highest binding energy per nucleon, meaning that fusion of iron nuclei would require an input of energy rather than releasing energy. As a result, fusion reactions cease beyond the formation of iron in the core of a star.
Learn more about photons here:- brainly.com/question/33017722
#SPJ11
the half life of N is 25 minutes. how long will it take 80% of the sample to decay
Answers
Explanation:
Nuclear half-life expresses the time required for half of a sample to undergo radioactive decay. Exponential decay can be expressed mathematically like this:
A
(
t
)
=
A
0
⋅
(
1
2
)
t
t
1/2
(1), where
A
(
t
)
- the amount left after t years;
A
0
- the initial quantity of the substance that will undergo decay;
t
1/2
- the half-life of the decaying quantity.
So, if a problem asks you to calculate an element's half-life, it must provide information about the initial mass, the quantity left after radioactive decay, and the time it took that sample to reach its post-decay value.
Let's say you have a radioactive isotope that undergoes radioactive decay. It started from a mass of 67.0 g and it took 98 years for it to reach 0.01 g. Here's how you would determine its half-life:
Starting from (1), we know that
0.01
=
67.0
⋅
(
1
2
)
98.0
t
1/2
→
0.01
67.0
=
0.000149
=
(
1
2
)
98.0
t
1/2
98.0
t
1/2
=
log
0.5
(
0.000149
)
=
12.7
Therefore, its half-life is
t
1/2
=
98.0
12.7
=
7.72
years
.
So, the initial mass gets halved every 7.72 years.
Sometimes, if the numbers allow it, you can work backwards to determine an element's half-life. Let's say you started with 100 g and ended up with 25 g after 1,000 years.
In this case, since 25 represents 1/4th of 100, two hal-life cycles must have passed in 1,000 years, since
100.0
2
=
50.0
g
after the first
t
1/2
,
50.0
2
=
25.0
g
after another
t
1/2
.
So,
2
⋅
t
1/2
=
1000
→
t
1/2
=
1000
2
=
500
years
.
Is it possible to get radiation from someone who has been exposed to radiation?
Answers
Answer: On the outside you can't spread radiation from person to person but if a person comes in contact with something radiated from the inside like urine or vomit then yes
During the work-up step, aqueous hydrochloric acid is added to the reaction and a gas is observed. what is the composition of the gas?
Answers
The gas likely composed of Hydrogen
Hydrogen is one of the most abundant elements that exist in the world and its natural state is a gaseous state. This means hydrogen can be in other states such as liquid or solid if bonded with other elements but it is not its natural state.
For example:
Ice is solid and it contains both hydrogen and oxygen
Hydrochloric acid is usually liquid and it contains Chlorine and Hydrogen
Because of this, during reactions Hydrogen has a tendency to break its bonds with other elements and return to its gaseous state. Based on this, it is likely the gas in this reaction is hydrogen.
Learn more in: https://brainly.com/question/11837837
A glass sphere is filled to full volume with a gas. The pressure of the gas inside the sphere is 30.0 atm, and the temperature is 30.0°C. The sphere is taken outside on a cold day. The temperature of the gas decreases to 10.0°C. What is the new pressure of the gas? Assume that the volume is constant.
Answers
Answer:
28atm
Explanation:
Using Gay lussac's law equation as follows:
P1/T1 = P2/T2
Where;
P1 = initial pressure (atm)
T1 = initial temperature (K)
P2 = final pressure (atm)
T2 = final temperature (K)
Based on the information provided in this question;
P1 = 30.0 atm, T1 = 30.0°C, P2 = ?, T2 = 10.0°C
NOTE: Absolute temperature i.e. Kelvin is required for this law
T1 = 30°C + 273K = 303K
T2 = 10°C + 273K = 283K
Using P1/T1 = P2/T2
30/303 = P2/283
Cross multiply
P2 × 303 = 30 × 283
303P2 = 8490
P2 = 8490/303
P2 = 28.02
New pressure of the gas = 28atm
Pb(NO3)2 (aq) + 2 NaI (aq) --> PbI2 (s) + 2 NaNO3 (aq)
Starting with with 200.0 grams of Pb(NO3)2 and 120.0 grams of NaI:
A. What is the limiting reagent?
B. How many grams of PbI2 is theoretically formed?
C. How many grams of the excess reactant remains?
D. If 48 grams of NaNO3 actually formed in the reaction, what is the percent yield of this reaction?
Answers
Starting with with 200.0 grams of Pb(NO3)2 and 120.0 grams of NaI:
A. What is the limiting reagent?
B. How many grams of PbI2 is theoretically formed?
C. How many grams of the excess reactant remains?
D. If 48 grams of NaNO3 actually formed in the reaction, what is the percent yield of this reaction?
HELP PLSSSSSS ASAPPPPP

Answers
My key
Synthesis Reactions = SR
Decomposition Reaction = DR
Single Replacement Reaction’s = SRR
Double Replacement Reactions = DRR
Combustion Reactions = CR
#1 SR
#2 SR
#3 SR
#4 SRR
#5 DRR
#6 SR
#7 DR
#8 DR
#9 sorry not sure
#10 DR
#15 SR
Why can no substance reach the temperature of absolute zero?
Because we do not yet have the technology to make something that cold
Because matter heats up too fast for it to get to absolute zero
Because something at absolute zero would have no kinetic energy, and atoms can never stop vibrating
Answers
Answer:
Because something at absolute zero would have no kinetic energy, and atoms can never stop vibrating.
Explanation:
If something were to reach absolute zero, that means that the particles would reach a position were we are able to detect precisely their velocity and position in the atom.
This cannot be possible because it would violate Heisenberg's Uncertainty Principle, which in brief states that we can measure the velocity of electron but we would be uncertain about the position and vice versa. We cannot predict both velocity and position accurately.
how many electrons are represented in the lewis electron dot structure of an element with 14 electrons and 14 protons in the neutral state?
Answers
4(four) electrons are represented in the Lewis electron dot structure of an element with 14 electrons and 14 protons in the neutral state
In the atom of an element, there are several levels of energy that are filled with electrons. The lowest energy level of the electrons is found in the innermost shell, while the greatest energy level is found in the outermost shell. Moving to a higher shell allows an electron to enhance its energy level, whereas moving to a lower shell causes it to reduce its energy level. It possesses an equal amount of positive and negative electric charges (the electrons) (the protons). Since of this, an atom is considered to be neutral because its overall electric charge is zero.
how many electrons are represented in the Lewis electron dot structure of an element with 14 electrons and 14 protons in the neutral state?
Learn more about electrons here:
https://brainly.com/question/1255220
#SPJ4