in an array vasked omplantation of a dictionary you can store key vakue pairs
Answers
The statement "In an array-based implementation of a dictionary, you can store key-value pairs" is true.
We can store key-value pairs in an array-based implementation of a dictionary because this type of data structure allows for efficient storage and retrieval of information using keys as unique identifiers for the corresponding values. The array will have a fixed size, and each element in the array will contain a key-value pair.
The keys and values can be of any data type, and the array can be sorted or unsorted depending on the implementation. Accessing and modifying elements in the array will involve searching for the key and then updating the associated value. Overall, an array implementation of a dictionary can be an efficient way to store and retrieve data if the size of the dictionary is relatively small and the keys are easy to search for.
Learn more about array-based implementation: https://brainly.com/question/31750702
#SPJ11
Related Questions
Question:
In a laboratory demonstration, a balloon filled with methane and oxygen was exposed to a
flame. The result was a brief, large flame. The students were asked to formulate an equation for
the reaction. One answer is below.
CH, + 0 = CO,
This equation is incorrect.
A. Explain how and why it is incorrect
B. What would the correct equation be, and how do you know that?

Answers
Answer:
The laboratory demonstration consists of the following;
The compounds present in the combustion reaction = Methane, CH₄ and Oxygen, O₂
The chemical equation for the combustion reaction is given as follows;
CH₄ + 2O₂ → CO₂ + 2H₂O
Therefore;
A. The equation given as CH₄ + O → CO₂ is not correct because;
1) Oxygen gas exist as diatomic molecules, O₂, and given that the experiment involves the mixture of gases, the oxygen gas present which can exist as a separate compound, should be represented as O₂
2) The number of oxygen molecules in the reaction is two rather than one
3) The product also includes two molecules of water (vapor) H₂O
B. The correct equation for the reaction should be given as follows;
CH₄ + 2O₂ → CO₂ + 2H₂O
B i) The constituents of the equation is obtained by the knowledge of the fact that the combustion reaction of an organic substance such as methane in the presence of oxygen yields, carbon dioxide and water (vapor)
The equation showing the relative amounts the reacting compounds is by balancing the basic equation of the combustion of methane in the presence of oxygen
Explanation:
A solution of pH 7 has
Group of answer choices
the same concentration of H+ and OH‑
more OH- than H+
no H+ and no OH-
more H+ than OH-
Answers
Answer: same concentration
Explanation:
H+ makes it more acidic, OH- makes is more basic. 7 is neutral so it has the same amount of both.
Determine the number of particles present in 1.55 mol of sodium.
Answers
Answer: 1024 sodium ions
Explanation: The mathematical equation, N = n × NA, can be used to find the number of atoms, ions or molecules in any amount (in moles) of atoms, ions or molecules: 10 moles of helium atoms = 10 × (6.022 × 1023) = 6.022 × 1024 helium atoms. 10 moles of sodium ions = 10 × (6.022 × 1023) = 6.022 × 1024 sodium ions. (that took long)
You move a 12-newton box 6 meters across the floor. How much work have you done?
Answers
The amount of work that has been done would be 72 Joules.
What is work done?The work done by a body can be defined as the amount of energy transferred from one body to another body. In other words, work is the product of force and the distance moved by the force.
This can be expressed mathematically as:
Work = force x distance moved by the force.
In this case:
Force = 12 newton
Distance = 6 meters
Work done = 12 x 6
= 72 NM or 72 Joules
In other words, if I move a 12 Newton box by 6 meters across the floor, the amount of work done would be 72 Joules.
More on work done can be found here: https://brainly.com/question/13662169
#SPJ1
1. Write down the ideal gas law 2. Calculate the volume in ft3 of 101 b mol of an ideal gas at 68∘F and 30 psia. 3. A gas consists of 2% N2,79%CH4 and 19%C2H6 at 120∘F and 13.8 psia. a. What is the partial pressure of each component? b. What is the volume fraction of each component? 4. What is the value of the ideal gas constant R if the pressure is to be expressed in atm, the temperature in Kelvin, the volume in cubic feet and the quantity of material in pound moles.
Answers
1. The ideal gas law is expressed as PV = nRT, where P represents pressure, V represents volume, n represents the number of moles of gas, R represents the ideal gas constant, and T represents temperature.
2. To calculate the volume in ft^3 of 101 b mol of an ideal gas at 68°F and 30 psia, we can use the ideal gas law equation. However, we need to convert the given temperature and pressure to the appropriate units. Once the conversion is done, we can substitute the values into the equation and solve for V.
3. For the gas mixture consisting of 2% N2, 79% CH4, and 19% C2H6 at 120°F and 13.8 psia, we can determine the partial pressure of each component and the volume fraction of each component. The partial pressure of a component is calculated by multiplying the total pressure by the corresponding percentage.
4. The value of the ideal gas constant R is 10.7316 (ft^3·atm)/(lb·mol·K).
1. The ideal gas law, PV = nRT, relates the pressure, volume, temperature, number of moles, and the ideal gas constant for an ideal gas.
2. To calculate the volume of 101 b mol of an ideal gas at 68°F and 30 psia, we first need to convert the temperature to Kelvin and the pressure to atm. The conversion for temperature is K = (°F + 459.67) × (5/9), and for pressure, 1 atm = 14.696 psia. Once the conversion is done, we can substitute the values into the ideal gas law equation and solve for V.
3. For the gas mixture, we can calculate the partial pressure of each component by multiplying the total pressure (13.8 psia) by the corresponding percentage (2%, 79%, and 19%). The volume fraction of each component can be obtained by dividing the volume of the component by the total volume of the mixture.
4. The value of the ideal gas constant R depends on the chosen units. To express pressure in atm, temperature in Kelvin, volume in cubic feet, and quantity in pound moles, the appropriate value for R is 10.7316 (ft^3·atm)/(lb·mol·K). This value allows for consistent units in the ideal gas law equation and ensures accurate calculations.
Please note that the provided explanations are general guidelines for solving the given problems. It is important to double-check the specific unit conversions and calculations for accurate results.
To know more about ideal gas law click here:
https://brainly.com/question/30458409
#SPJ11
Which statement describes the components of a mixture?
A)
Each component gains new properties.
B)
Each component loses its original properties.
C
The proportions of components can vary.
D)
The proportion of components cannot vary.
Answers
Answer:c
Explanation: the proportions of components can vary
Yooo I’m tryna get some ppls snap so we make a gc and all play among us together
Answers
Answer:
lilpeep66661
Explanation:
add me i play
What is function of phylloclade
Answers
In some plants such as Cactuses, the stem is flattened and green and carries out the function of the leaf. Such a stem, adapted for the manufacture of food is called phylloclade. The leaves here are reduced or modified into spines to lessen the transpiring surface.
Write down your observations of what the pieces of cabbage in the images look like

Answers
when all the electrons in an atom occupy the lowest possible energy states, the atom is said to be in its:
Answers
when all the electrons in an atom occupy the lowest possible energy states, the atom is said to be in its Ground state.
A quantum mechanical system's ground state is its stationary, lowest energy state; this energy is often referred to as the system's zero-point energy. Any state that has more energy than the ground state is said to be excited. The ground state is frequently referred to as the vacuum state or the vacuum in quantum field theory.
They are referred to as degenerate if they have more than one ground state. There are degenerate ground states in many systems. Degeneracy arises whenever a unitary operator exists that interacts nontrivially with a ground state and commutes with the system's Hamiltonian.
The third law of thermodynamics states that a system is in its ground state at absolute zero degrees Celsius, and that ground state's degeneracy determines the system's entropy. At absolute zero, many systems, including a flawless crystal lattice, have a singular ground state and so have zero entropy. In systems that display negative temperature, it is also conceivable for the most excited state to have absolute zero temperature.
For more such questions on Ground state, Visit:
https://brainly.com/question/16908172
#SPJ4
What ion does sodium (Na) form
Answers
Explained briefly
Which of the following is most critical for maintaining the native tertiary structure of a globular protein? Hydrogen bonds between regions of the polypeptide chain in the folded protein The hydrophobic effect of burying non-polar residues in the protein interior. Disulfide bonds between Cys residues in the protein. Salt bridges (i.e. ionic bonds between opposite charged side chains). None of these choices.
Answers
Among the given options, hydrogen bonds between regions of the polypeptide chain are the most critical for maintaining the native tertiary structure of a globular protein. They provide specific and directional interactions, contributing significantly to the stability of the folded protein.
The most critical factor for maintaining the native tertiary structure of a globular protein is the hydrogen bonds between regions of the polypeptide chain in the folded protein. Here's why:
1. Hydrogen bonds are formed between the electronegative atoms, typically oxygen or nitrogen, and hydrogen atoms in the protein. They contribute to the stabilization of the folded structure by providing specific and directional interactions between different regions of the polypeptide chain.
2. The hydrophobic effect, which involves the burying of non-polar residues in the protein interior, is important for stabilizing the protein's overall structure, but it does not directly contribute to maintaining the native tertiary structure.
3. Disulfide bonds between cysteine (Cys) residues can form covalent bonds and play a critical role in stabilizing the tertiary structure of some proteins, especially those found in extracellular environments. However, not all proteins have disulfide bonds, and the absence of disulfide bonds does not necessarily lead to loss of the native tertiary structure.
4. Salt bridges, also known as ionic bonds, occur between oppositely charged side chains and contribute to the stabilization of protein structure. While they can play a role in maintaining the structure, they are generally less critical compared to hydrogen bonds.
Learn more About hydrogen bonds from the given link
https://brainly.com/question/1426421
#SPJ11
A sample of oxygen gas has a pressure of 6.58 kPa at 539 K. If the volume does not change, what will be the pressure at -62.0°C?
Answers
Given that the volume remains constant, the sample of oxygen gas will have a pressure of 10.67 kPa at a temperature of -62.0°C, according to the question.
What is pressure?The force per unit area applied to a surface is described by the fundamental physical quantity known as pressure. It is quantified in units of force like pounds per square inch (psi) or newtons per square metre (N/\(m^2\)). In addition to measuring the amount of force delivered to an area, pressure can also be used to gauge how much work a system has accomplished.
Because a gas sample's pressure and temperature are inversely correlated, when the temperature varies, so does the gas pressure. The Ideal Gas Law, which states that P*V = n*R*T, where P is pressure, V is volume, n is the number of moles, R is the ideal gas constant, and T is temperature, describes the relationship between pressure and temperature for a certain volume of gas.
As a result, we may modify the Ideal Gas Law equation to solve for P in order to determine the pressure at -62.0°C:
P = (n*R*T) / V
P = (n*R*(-62.0 + 273.15)) / V
P = (n*R*211.15) / V
P = (6.58 kPa * 8.314 J/K·mol * 211.15 K) / V
P = 10.67 kPa
Given that the volume remains constant, the pressure of the sample of oxygen gas will be 10.67 kPa at -62.0°C.
To learn more about pressure
brainly.com/question/24719118
#SPJ1
How many CO2 molecules are in 1 mole of carbon dioxide?
Answers
Answer:
I guess 1-mole carbon dioxide contains 6.02 x 10^23 molecules.
When using silica gel or alumina, increasing the polarity of the eluent will __________________the rate that a polar compound passes through the column.
Answers
When using silica gel or alumina, increasing the polarity of the eluent will increase the rate that a polar compound passes through the column.
Silica gel and alumina are both polar stationary phases. In chromatography, the interaction between the stationary phase and the analyte determines the retention of the analyte on the column. Polar compounds will have stronger interactions with these polar stationary phases compared to non-polar compounds.
The eluent, or mobile phase, is responsible for carrying the analyte through the column. When the polarity of the eluent is increased, it competes more effectively with polar analytes for interactions with the polar stationary phase. As a result, polar analytes are less retained and move through the column more rapidly.
In summary, increasing the polarity of the eluent in column chromatography using silica gel or alumina as stationary phases leads to a faster migration of polar compounds through the column. This occurs because the polar eluent reduces the interaction strength between the polar analyte and the polar stationary phase, allowing the analyte to move more quickly through the column.
Know more about Polar compound here :
https://brainly.com/question/29340665
#SPJ11
The helium-filled blimp shown in fig. p1.34 is used at various athletic events. determine the number of pounds of helium within it if its volume is 68,000 ft3 and the temperature and pressure are 80 f and 14.2 psia, respectively.
Answers
Answer:
The number of pounds of helium n = 666.995 lbm
Explanation:
Given that :
the volume V = 68000 ft³
the temperature T = 80 f = (80 + 460)°R =540°R
the pressure P = 14.2 psia
Using the ideal gas equation PV =nRT to determine the number of pounds of helium within, we have :
n = PV/RT
where;
R = 2.6809 psia. ft³/lbm.R (from the tables of A-1E for helium)
n = (14.2 × 68000)/(2.6809 × 540)
n = 965600/1447.686
n = 666.995 lbm
∴
The number of pounds of helium n = 666.995 lbm
A car accelerates from 10 km/h to 80 km/h in 8.0 seconds. Determine the car’s acceleration in m/s2.
Answers
Answer:
The difference in velocity divided by rate = acceleration.
80 - 10 = 70km/h
60 sec. per hour, so 70 × 60 = 4,200 km/s
4.200/8=525
520/60 = 8.6666666667 km/h
Explanation:
Not the answer but hope it puts you in the neighborhood.
The resultant acceleration of the car is 2.43 m/s².
What is acceleration?Acceleration is define as the rate of change of velocity with respect to the time and it will be calculated as:
Acceleration = Velocity / Time
Given that, velocity of car = 80km/h - 10km/h = 70km/h
As we know that 1 km = 1000m
and 1 hour = 3600 sec
So that 70km/h = 19.4 m/s
Given time = 8 sec
Now putting values on the acceleration equation, we get
Acceleration = 19.4 m/sec / 8sec = 2.43 m/s²
Hence required acceleration is 2.43 m/s².
To know more about acceleration, visit the below link:
https://brainly.com/question/605631
#SPJ2
PLEASE HELPPP AND THANK YOUUUU
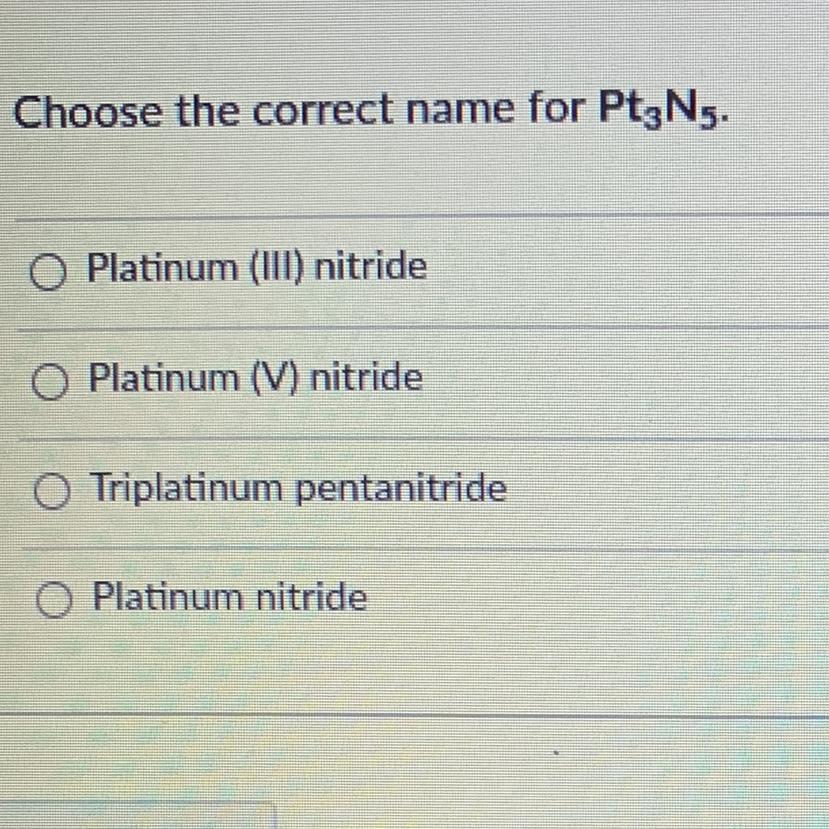
Answers
if pressure is kept constant, to compress nitrogen at 2 atm from 750 ml to 500 ml, what must the new pressure be?
Answers
The new pressure be if pressure is kept constant, to compress nitrogen is 3 atm.
Why Boyle's Law is important?Boyle's law is significant because it explains how gases behave. It explains with certainty that the relationship between gas pressure and volume is inverse. So, when you exert pressure on gas, the volume decreases and its pressure increases.
Briefing:P1V1 = P2V2
P1 and V1 are initial pressure and volume.
P2 and V2 are final pressure and volume.
P1 = 1 atm
V1 = 750 mL
P2 = ?
V2 = 500 mL
Putting values
2 * 750 mL = P2 * 500 mL
P2 = 3 atm
To know more about Boyle's law visit:
https://brainly.com/question/1437490
#SPJ4
Help me with this plz

Answers
Answer:
3.6 moles
Explanation: i got that quistion right .
stomach acid is about 0.155 m hcl and has about the same density as water. did the rolaids tablet neutralize 47 times its own weight of hcl solution?
Answers
1 Rolaids tablet can neutralize 235 mg of HCl, the calculations are shown in the below section.
First, we have to find the amount of acid (HCl) that is present in the stomach. It is estimated that about 0.16 M HCl is present in the stomach, which is produced by parietal cells. It is clear that the density of HCl is similar to that of water. Hence, the density of HCl is taken as 1 g/mL. Now we need to find out the amount of HCl that is present in the solution. Let's calculate it.
Molarity = moles / volume (L)
0.155 = moles / 1 mole/1000 mL
moles = 0.155 × 1/1000 = 1.55 × 10⁻⁴ mol
Number of moles of HCl in 1000 mL of HCl solution is 1.55 × 10⁻⁴ mol.
Now let's calculate the mass of HCl in 1000 mL of the solution.
Molar mass of HCl = 36.5 g/mol mass of 1.55 × 10⁻⁴ mol of HCl = 36.5 × 1.55 × 10⁻⁴ = 0.005 m g
Volume of 1 Rolaids tablet = 20 mL
Density of Rolaids tablet = 1.6 g/mL
Mass of 1 Rolaids tablet = density × volume = 1.6 g/mL × 20 mL = 32 g
Mass of HCl neutralized by 1 Rolaids tablet is 47 times its own weight of HCl solution.
Hence, the amount of HCl neutralized by 1 Rolaids tablet is 0.005 × 47 = 0.235 g = 235 mg.
This means that 1 Rolaids tablet can neutralize 235 mg of HCl.
To learn more about Density check the link below-
https://brainly.com/question/1354972
#SPJ11
a water molecule can bond to up to other water molecules by bonds. a water molecule can bond to up to other water molecules by bonds. two ... hydrogen three ... ionic four ... polar covalent two ... polar covalent four ... hydrogen
Answers
A water molecule can bond to up to 3 other water molecules by hydrogen bonding.
What is hydrogen bond ?A hydrogen bond, also known as an H-bond, is an attraction that is essentially electrostatic and occurs when an electronegative atom with a single pair of electrons, or the hydrogen bond acceptor, is covalently attached to a greater electronegative "donor" atom or group (Dn) (Ac). A polar covalent bond is often denoted by a solid line, while a hydrogen bond is denoted by a dotted or dashed line. Such an interacting system is generally represented by the symbols DnHAc. The second-row elements nitrogen (N), oxygen (O), and fluorine (F) are used as donors and acceptors the most frequently (F).Intermolecular (between different molecules) or intramolecular (occurring among parts of the same molecule) hydrogen bonds are two different types of hydrogen bonding .Learn more about Hydrogen bond with the given link
https://brainly.com/question/16854739
#SPJ4
Explain why mass is used to measure the properties of solids, liquids, and gases.
Answers
Answer: Mass takes into account the force of gravity.
Explanation:
What conclusion can you draw about the ability of metals to hold on to and attract electrons, as
compared to nonmetals?
Answers
Answer:
Metals react by losing electrons. So, there is high reactivity due to lower attraction. Non-metals react by gaining electrons. So, there is high reactivity due to higher attraction.
Explanation:
Metals react by losing electrons. So, there is high reactivity due to lower attraction. Non-metals react by gaining electrons. So, there is high reactivity due to higher attraction. Also, electrons lost by metals transfer to the nonmetals. It is easier for the metals to lose their valance electrons and form cations rather than gaining electrons.
Metals do not hold on to or attract electrons while nonmetals hold on to or attract electrons.
In the periodic table, metals are found towards the left hand side of the table while nonmetals are found towards the right hand side of the table.
Electron affinity of elements increase from left to right across the period. Electron affinity refers to the ability of elements to attract or hold electrons. This ability increase steadily across the period.
Usually, the electron affinity values of nonmetals are very high showing that they easily hold on to and attract electrons while the electron affinity values of metals is very low showing that they do not easily hold on to and attract electrons.
Learn more: https://brainly.com/question/3964366
Which of the following is a synthesis reaction?
AgNO3 + NaCl → AgCl + NaNO3
CH4 + O2 → CO2 + H2O
SO3 + H2O → H2SO4
Cu + AgNO3 → Ag + CuNO3
Answers
Synthesis reaction
It is a reaction in which 2 or more reactants combine with each other to form one product .
Check option C
Sulphate and water are combining to form sulfuric acid .
Hence option C is correct
Answer:
SO3 + H2O => H2SO4
Explanation:
I took the test :)
Question 14 PM2.5 is defined as ________
- the mass concentration of particles in the air less than or equal to 2.5 micrometers in diameter. - the mass concentration of particles in the air equal to 2.5 micrometers in diameter. - the mass concentration of particles in the air greater than or equal to 2.5 micrometers in diameter. Question 15 Carbon dioxide (CO2) is a criteria air pollutant. - True - False Question 16 Roughly percent of emissions of carbon monoxide in Santa Clara County come from mobile sources (select the choice closest to the correct answer). - 50 - 75 - 25 Question 17
The term "photochemical smog" is most synonymous with which of the following criteria air pollutants? - lead (Pb) - carbon monoxide (CO) - sulfur dioxide ( SO2) - ozone (O3) Question 18 "Attainment" of ambient air quality standards requires that measured concentrations at all monitoring stations within an air district are below ambient air standards. - True - False
Answers
: PM2.5 is defined as the mass concentration of particles in the air less than or equal to 2.5 micrometers in diameter.Question 15: False, carbon dioxide (CO2) is not considered a criteria air pollutant.
Question 16: The closest answer is 50%, but the exact percentage is not provided in the question.Question 17: The term "photochemical smog" is most synonymous with ozone (O3), which is a criteria air pollutant.Question 18: True, attainment of ambient air quality standards requires that measured concentrations at all monitoring stations within an air district are below ambient air standards.
Question 14 asks about the definition of PM2.5. PM2.5 refers to particulate matter with a diameter less than or equal to 2.5 micrometers. It represents the mass concentration of particles suspended in the air, which are small enough to be inhaled into the respiratory system and can have adverse health effects.
Question 15 states whether carbon dioxide (CO2) is a criteria air pollutant. Criteria air pollutants are a set of pollutants regulated by environmental agencies due to their detrimental impact on air quality and human health. However, carbon dioxide is not considered a criteria air pollutant because it does not directly cause harm to human health or the environment in the same way as pollutants like ozone or particulate matter.
Question 16 asks about the percentage of carbon monoxide (CO) emissions from mobile sources in Santa Clara County. While the exact percentage is not provided in the question, the closest answer option is 50%. However, it is important to note that the precise percentage may vary depending on specific local conditions and emissions sources.
Question 17 inquires about the criteria air pollutant most synonymous with the term "photochemical smog." Photochemical smog is primarily associated with high levels of ground-level ozone (O3). Ozone is formed when nitrogen oxides (NOx) and volatile organic compounds (VOCs) react in the presence of sunlight, creating a hazy and polluted atmospheric condition.
Question 18 addresses the concept of "attainment" of ambient air quality standards. To achieve attainment, measured concentrations of pollutants at all monitoring stations within an air district must be below the established ambient air quality standards. This ensures that the air quality in the given area meets the required standards for protecting human health and the environment.
Learn more about mass concentration here:- brainly.com/question/23437000
#SPJ11
QUESTION 4 An aqueous solution of 4 mol/L nitric acid is electrolyzed in an electrolytic cell using graphite electrodes.
a) Write the chemical symbols for all the ions present in the electrolytic cell. (1 mark]
b) Name the gas given off at the cathode. [1 mark]
c) Name the gas given off at the anode. [1 mark]
d) Write the half reaction equations at the;
i) Cathode : [1 mark)
ii) Anode : [1 mark]
e) The overall anode - cathode reaction equation taking place in the electrolytic cell. [1 mark]
Answers
The gas given off at the cathode is hydrogen while the gas given off at the anode is oxygen.
What is electrolysis?The term electrolysis has to do with the decomposition of a substance by the passage of direct current through it.
The ions present in the system are H^+, OH^- and NO3^- ions. The gas given off at the cathode is hydrogen while the gas given off at the anode is oxygen.
The half reaction equation of the reduction is;
2H^+(aq) + e ----->H2(g)
The oxidation half reaction equation is;
4OH^- (aq)------> 2H2O(l) + 4e
The overall equation is;
4H^+(aq) + 4OH^- (aq)------> 4H2(g) + 2H2O(l) + 4e
Learn more about electrolysis:https://brainly.com/question/12054569
#SPJ1
2) the initial reactants in this lab are aluminum metal and potassium hydroxide. which is the limiting reactant? explain your answer.
Answers
Al2 (SO4)3 + H2 + H2SOy 2 Al mass = 1.105 g,
2 Al molar mass = 26.98 g/mol,
and 2 Al moles = 1.105 / 26.98 = 0.041 moles.
KOH concentration is 1.4 M.
KOH's volume is 52.2 mL, or 0.0522 L, and its moles are 1.4 x 0.0522, or 0.07308 moles.
2Al + 2 KOH + 4 H2SO4 + 22H2O2KAI concentration of H2SO4 = 9.0 M volume of H2SOy = 21.8 ml = 0.0218 L moles of H2SO4 = 9.0 X 0.0218 = 0.1962 moles (SO4).
Aluminum contains the fewest number of moles, 12 H2O + 3 H2.
Reagent is constrained by aluminum.
A 2 mole Al creates a 3 mole H2 from the aforementioned reaction. In other words, 0.041 moles of Al generate 0.041 x 1.5 moles of H2, or 0.0615 moles of H2.
H2 quantity equals moles times molar mass.
= 0.0615 x 2g
Amount of H₂ produced = 0.123g
To know more about limiting reactant, click on the link below:
https://brainly.com/question/26905271
#SPJ4
A current is passed through three electrolytic cells connected in series containing solutions of silver trioxonitrate (v), copper (II)tetraoxosulphate (VI) and brine respectively. If 12.7g of copper are deposited in the second electrolytic cell, calculate :
A: the mass of silver deposited in the first cell
B:the volume of chlorine liberated in the third cell at 17°c and 800mmhg pressure.
(Ag=108,cu=63.5,1F=96500c,GMV of gas at S.T.P=22.4dm^3)?
Answers
Answer: check out the image
Explanation: check out the image


what is a mixture of elements and compounds

Answers
The substance in the image above would be classified as a mixture of elements (option E).
What is a compound and mixture?A compound is a substance formed by chemical bonding of two or more elements in definite proportions by weight.
On the other hand, a mixture is made when two or more substances are combined, but they are not combined chemically.
According to this question, an image is shown with two different substances or elements as distinguished by coloration (white and purple). These elements are combined but not chemically bonded, hence, is a mixture.
Learn more about mixture at: https://brainly.com/question/12160179
#SPJ1