Answers
Answer:
\(H_2O\)Explanation:
Here, we want to get the type of substances which are acids according to the Bronsted-Lowry definition
According to their definition, an acid donates or is the source of H+ ions in an acid-base reaction
Let us look at the reactants, we can see that while the ammonia molecule accepted a donation of hydrogen ion to become Ammonium ion, the water molecule lost a hydrogen ion to become the hydroxide ion
Thus, we can conclude here that the water molecule is the acid in the reaction
Related Questions
Which phrase best describes nuclear fusion?(1 point)
the process by which small nuclei combine into a larger nucleus
a series of reactions in which particles from one reaction trigger the next reaction
the process by which a large nucleus is divided into smaller nuclei
the spontaneous emission of radiation from an unstable nucleus
Which phrase describes a nuclear chain reaction?
a reaction in which small nuclei combine into a larger nucleus
a reaction in which particles spontaneously emit radiation
a type of reaction which only takes place in the sun and other stars
a series of reactions in which particles from one reaction trigger the next reaction
Which option would be an appropriate model of nuclear fission?(1 point)
taking a teaspoon of sugar from a bowl
cutting a cake in half
disturbing a drop of water such that it breaks into smaller droplets
dividing a stack of paper equally between two people
The process of radioactive decay is unpredictable and irreversible. Which option would be an appropriate model of the radioactive decay of a group of atoms?
petals falling from a flower
throwing tennis balls over a fence
breaking off pieces of wet clay
popping a bag of popcorn
Which statement best describes the effect of radioactive decay on a nucleus?
The resulting nucleus is more stable than the original nucleus. The nucleus can be of a different element than the original.
The resulting nucleus is more stable than the original nucleus. The nucleus must be of the same element as the original.
The resulting nucleus is less stable than the original nucleus. The nucleus can be of a different element than the original.
The resulting nucleus is less stable than the original nucleus. The nucleus must be of the same element as the original.
Answers
Answer:
b
Explanation:
Answer:
B
Explanation:
Took the test
What is the percent of C in Ca(C2H302)2? (Ca = 40.08 gkmol, C = 12.01 g/mol, H= 1.01 g/mol, O = 16.00 g/mol) [?1%C Round your answer to the hundredths place. [?] % C
Answers
Answer:
Ca(C2H3O2)2 has 30.41% carbon by volume
Explanation:
How many grams of O2 are present in 44.1 L of O2 at STP?
a.10.0 g
b.16.0 g
c.32.0 g
d.410.0 g
e. 63.0 g
Answers
Taking into accoun the STP conditions and the ideal gas law, the correct answer is option e. 63 grams of O₂ are present in 44.1 L of O2 at STP.
First of all, the STP conditions refer to the standard temperature and pressure, where the values used are: pressure at 1 atmosphere and temperature at 0°C. These values are reference values for gases.
On the other side, the pressure, P, the temperature, T, and the volume, V, of an ideal gas, are related by a simple formula called the ideal gas law:
P×V = n×R×T
where:
P is the gas pressure.V is the volume that occupies.T is its temperature.R is the ideal gas constant. The universal constant of ideal gases R has the same value for all gaseous substances.n is the number of moles of the gas.Then, in this case:
P= 1 atmV= 44.1 Ln= ?R= 0.082 \(\frac{atmL}{molK}\)T= 0°C =273 KReplacing in the expression for the ideal gas law:
1 atm× 44.1 L= n× 0.082 \(\frac{atmL}{molK}\)× 273 K
Solving:
\(n=\frac{1 atm x44.1 L}{0.082\frac{atmL}{molK}x273K}\)
n=1.97 moles
Being the molar mass of O₂, that is, the mass of one mole of the compound, 32 g/mole, the amount of mass that 1.97 moles contains can be calculated as:
\(1.97 molesx\frac{32 g}{1 mole}\)= 63.04 g ≈ 63 g
Finally, the correct answer is option e. 63 grams of O₂ are present in 44.1 L of O2 at STP.
Learn more about the ideal gas law:
https://brainly.com/question/4147359?referrer=searchResultswhich one is not allotropes of carbon?

Answers
Answer:
B. Activated Carbon
Explanation:
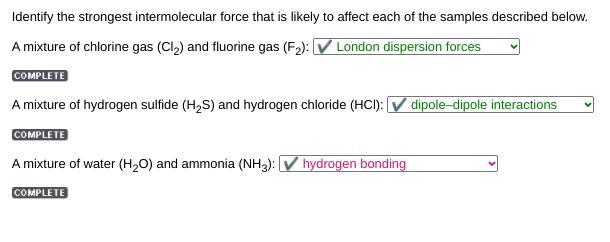
Identify the strongest intermolecular force that is likely to affect each of the samples described below.
A mixture of chlorine gas (Cl) and fluorine gas (F): V London dispersion forces
COMPLETE
Tweaks
Menu
A mixture of hydrogen sulfide (H2S) and hydrogen chloride (HCI): V dipole-dipole interactions
Search
Selection
COMPLETE
Guess
this
hydrogen bonding
A mixture of water (H2O) and ammonia (NH3):
Answers
Answer:
A mixture of chlorine gas (Cl2) and fluorine gas (F2):
✔ London dispersion forces
Explanation:
Which of these is NOT true about sandy soil?
A) It's gritty to the touch.
B) It crumbles, even when wet.
C) It's made of large particles.
D) It supports many kinds of plants.
Answers
ALEKS Ammonia will decompose into nitrogen and hydrogen at high temperature. An industrial chemist studying this reaction fills a 5.0L flask with 2.7 atm of ammonia gas, and when the mixture has come to equilibrium measures the amount of nitrogen gas to be 0.54 atm.
Required:
Calculate the pressure equilibrium constant for the decomposition of ammonia at the final temperature of the mixture.
Answers
2NH3(g) = N2(g) + 3H2(g)
Before decomposed :
P NH3 = 2.7 atm
After decomposed :
P N2 = 0.54 atm
P H2 = P N2 / 3 = 0.54 / 3 = 0.18 atm
P NH3 = 2.7 - 2(0.18) = 2.34 atm
Pressure equilibrium constant :
Kp = (P N2)(P H2)³ / (P NH3)²
Kp = (0.54)(0.18)³ / (2.34)²
Kp = 5.75 × 10^(-4)
61. Given the following information:
Ag2 CrO4(s)=2Agt (aq) + CrO4²- (aq)
Ag+ (aq) + e- Ag(s)
find the standard reduction potential at 25°C for the half-reaction
Ksp = 1 × 10-12
E = +0.799 V
Ag2 CrO4(s) + 2e¯ 2Ag(s) + CrO4²- (aq)
Answers
Q = Ksp = 1 × 10^(-12).
Substituting the values into the Nernst equation, we have:
0.799 V = E° - (RT/2F) * ln(1 × 10^(-12))
Now, solving for E°:
E° = 0.799 V + (RT/2F) * ln(1 × 10^(-12))
The value of R is the ideal gas constant, T is the temperature in Kelvin, and F is the Faraday constant.
To find the standard reduction potential at 25°C for the half-reaction Ag2CrO4(s) + 2e¯ → 2Ag(s) + CrO4²-(aq), we can use the Nernst equation, which relates the standard reduction potential (E°) to the equilibrium constant (K) and the reaction quotient (Q).
The Nernst equation is given as follows:
E = E° - (RT/nF) * ln(Q)
Given information:
Ksp = 1 × 10^(-12)
E = +0.799 V (standard reduction potential of Ag+ to Ag)
Since the reaction involves the dissolution of Ag2CrO4(s), the reaction quotient Q can be expressed as [Ag+]²/[CrO4²-].
Since the stoichiometry of the reaction is 2:1 for Ag2CrO4 to Ag+, we can say that [Ag+]² = Ksp.
Therefore, Q = Ksp = 1 × 10^(-12).
Substituting the values into the Nernst equation, we have:
0.799 V = E° - (RT/2F) * ln(1 × 10^(-12))
Now, solving for E°:
E° = 0.799 V + (RT/2F) * ln(1 × 10^(-12))
The value of R is the ideal gas constant, T is the temperature in Kelvin, and F is the Faraday constant.
Please note that without specific values for temperature (T) and the ideal gas constant (R), the exact standard reduction potential at 25°C cannot be determined.
For more question on temperature
https://brainly.com/question/4735135
#SPJ8
what element has Atom of 47
Answers
Isabel and Ruth scored a total of twenty-two points in their last basketball game scored two more points than Isabelle.How many points did Isabel score?
A:2x-2=22; x=12
B:x+2=22; x=20
C:2x+2=22; x=10
D:2x+2=22; x=12
Answers
isabel is represented as x
ruth is represented as x+2
so the equation would be
x+(x+2)=22
the (x+2) is ruth. so to check if its right solve it. 2x+2=22
-2 -2
2x+20
__ ___
2 2
x=10
so now you know that isabel scored 10 goals since she was x. to figure out ruth just add 2.
2H+O2 → 2H₂O
Three grams of hydrogen and 24 grams of oxygen were completely reacted to form
water. Which of the following is the amount of water produced?
Answers
27 grams of water are produced from the given amounts of hydrogen and oxygen.
To determine the amount of water produced from the reaction between hydrogen and oxygen, we need to compare the amounts of reactants (hydrogen and oxygen) to the stoichiometry of the balanced chemical equation.
The balanced equation is:
2H₂ + O₂ → 2H₂O
The molar mass of hydrogen (H₂) is 2 g/mol, and the molar mass of oxygen (O₂) is 32 g/mol.
Given:
Mass of hydrogen = 3 grams
Mass of oxygen = 24 grams
We can calculate the number of moles of each reactant by dividing their respective masses by their molar masses:
Number of moles of hydrogen = 3 grams / 2 g/mol = 1.5 moles
Number of moles of oxygen = 24 grams / 32 g/mol = 0.75 moles
According to the balanced equation, the ratio of hydrogen to water is 2:2, and the ratio of oxygen to water is 1:2. Therefore, the limiting reactant in this reaction is oxygen, as it is consumed in a smaller quantity compared to the stoichiometry of the reaction.
From the reaction, we can see that 1 mole of oxygen produces 2 moles of water. Thus, the number of moles of water produced is twice the number of moles of oxygen:
Number of moles of water = 2 * 0.75 moles = 1.5 moles
To determine the amount of water produced in grams, we multiply the number of moles by the molar mass of water (H₂O), which is approximately 18 g/mol:
Mass of water produced = 1.5 moles * 18 g/mol = 27 grams
for more such questions on water
https://brainly.com/question/19491767
#SPJ11
Net ionic equation for potassium sulfide and magnesium iodide
Answers
The net ionic equation for the reaction between potassium sulfide and magnesium iodide is S2- + Mg2+ -> MgS, as the potassium and iodide ions are spectator ions and do not participate in the reaction.
To determine the net ionic equation for the reaction between potassium sulfide (K2S) and magnesium iodide (MgI2), we first need to identify the ions present in each compound and then determine the products formed when they react.
Potassium sulfide (K2S) dissociates into two potassium ions (K+) and one sulfide ion (S2-):
K2S -> 2K+ + S2-
Magnesium iodide (MgI2) dissociates into one magnesium ion (Mg2+) and two iodide ions (I-):
MgI2 -> Mg2+ + 2I-
Now, we need to determine the possible products when these ions combine. Since potassium (K+) has a +1 charge and iodide (I-) has a -1 charge, they can combine to form potassium iodide (KI):
K+ + I- -> KI
Similarly, magnesium (Mg2+) and sulfide (S2-) can combine to form magnesium sulfide (MgS):
Mg2+ + S2- -> MgS
Now, we can write the complete ionic equation by representing all the ions present before and after the reaction:
2K+ + S2- + Mg2+ + 2I- -> 2KI + MgS
To obtain the net ionic equation, we remove the spectator ions, which are the ions that appear on both sides of the equation and do not participate in the actual reaction. In this case, the spectator ions are the potassium ions (K+) and the iodide ions (I-).
Thus, the net ionic equation for the reaction between potassium sulfide and magnesium iodide is:
S2- + Mg2+ -> MgS
For more such questions on ionic equation visit:
https://brainly.com/question/25604204
#SPJ8
Please help my last question thank you if u did answer it


Answers
Answer:
I don't know sorry I hope you find what you're looking for
Answer:
I think the answer is G
Explanation:
Carbon atom has 6 electrons 6 protons and 6 neutrons.(electron =negetive) (proton=positive) (neutron=neutral)
You should know protons and neutrons are in nucleus of atom and electrons surround them.I think you understand.
0.487 grams of quinine (molar mass = 324 g/mol) is combusted and found to produce
1.321 g CO2, 0.325 g H2O and 0.0421 g nitrogen. Determine the empirical and molecular
formulas.
Answers
molar mass of empirical formula = (20 x 12.01 g/mol) + (24 x 1.01 g/mol) + (1 x 14.01 g/mol) = 324.44 g/mol ratio = 324.44 g/mol / 324 g/mol = 1.001. The molecular formula of quinine is C20H24N.
What is used for quinine?The active component of cinchona extracts, which have been used for this purpose since before 1633, is utilized as an antimalarial medication. Quinine has been utilized in conventional cold remedies for its use as a mild antipyretic and analgesic.
What drug is quinine?Plasmodium falciparum malaria is treated with quinine. Malaria is brought on by the parasite Plasmodium falciparum, which enters the body through the red blood cells. Quinine functions by either eliminating the parasite or halting its growth.
to know more about quinine here;
brainly.com/question/17275075
#SPJ1
Find the amount of heat (Q) needed to raise the temperature of 5.00g of a substance from 20C to 30C if the specific heat of the substance is 2.01J/gC.
Answers
Answer
100.5 J
Explanation
Given:
Mass of the substance, m = 5.00 g
Temperature change, ΔT = 30 ⁰C - 20 ⁰C = 10 ⁰C
Specific heat of the substance, c = 2.01 J/g⁰C
What to find:
The amount of heat (Q) needed to raise the temperature.
Step-by-step solution:
Using Q = mcΔT
The amount of heat (Q) needed to raise the temperature can be calculated.
Q = 5.00 g x 2.01 J/g⁰C x 10 ⁰C
Q = 100.5 J
Hence, the amount of heat (Q) needed to raise the temperature is 100.5 J
Which species has 54 electrons
Answers
Answer: Neutral Xe atom
_C₂H₄+ _ O₂ → _ CO₂ + _ H₂O If you start with 14.5 grams of ethylene (C₂H₄), how many grams of water(H₂O) will be produced?
Answers
Answer:
a. SOLUTION:
Step 1: Write the balanced chemical equation.
C₂H₄ + 3O₂ → 2CO₂ + 2H₂O
Step 2: Calculate the number of moles of CO₂ formed by each reactant.
• Using C₂H₄
Based on the balanced chemical equation, 1 mole of C₂H₄ is stoichiometrically equivalent to 2 moles of CO₂.
The molar mass of C₂H₄ is 28.054 g/mol.
• Using O₂
Based on the balanced chemical equation, 3 moles of O₂ is stoichiometrically equivalent to 2 mole of CO₂.
The molar mass of O₂ is 31.998 g/mol.
Step 3: Determine the limiting reagent.
Since O₂ produced less amount of CO₂ than C₂H₄, O₂ is the limiting reagent.
Step 4: Determine the mass of CO₂ formed.
Note that the (maximum) mass of a product that can be formed is dictated by the limiting reagent. In this case, we will start at the number of moles of CO₂ formed from the limiting reagent (O₂) which is equal to 0.11022 mol.
The molar mass of CO₂ is 44.009 g.
Hence, 4.85 g of CO₂ can be formed.
------------------------------------------------------------
b. ANSWER:
The LR is O₂ and the ER is C₂H₄.
------------------------------------------------------------
c. SOLUTION:
The theoretical yield of the reaction is 4.85 g.
Hence, the percent yield of the reaction is 87.6%.
#CarryOnLearning
Explanation:
brainliest pls
Which amphibian organ has a high blood supply and many folds to increase surface area?
a. heart
b. stomach
c. lungs
d. brain
Answers
Answer:
lungs
Explanation:
How much energy is required to melt a 250 gram ice cube?


Answers
Answer
Energy required to melt ice (Q) = 83500 j
Explanation
Given
Mass of ice = 250 g
Required: Energy to melt ice
Solution
Ice melts at B, where the temperature is 0 °C.
To solve this question, we will use:
\(Q\text{ = L}_{ice}\text{ x m}\)where Q is the amount of heat needed to melt the ice, L ice is the Latent Heat (or heat of fusion of water = 334 j/g) of melting for ice, and m is the given mass of ice.
Q = 334 j/g x 250 g
Q = 83500 j
Formal Write Up Lab 2 Double Replacement Reactions
Answers
A double replacement reaction occurs when components of two ionic compounds are swapped, resulting in the formation of two new compounds.
The double replacement reaction is also known as the double displacement, exchange, or metathesis reaction. The following is the general form of all double replacement reactions: AB+CD→AD+CB.
Precipitation reactions, neutralization reactions, and gas-forming reactions are examples of double-replacement reactions.
Examples of double replacement reactions:
1) Example of the precipitation reaction
Pb(NO3)2 (aq) + 2 NaCl (aq) → 2 NaNO3 (aq) + PbCl2
The anticipated byproducts include sodium nitrate and insoluble lead (II) chloride (soluble). A precipitation reaction will occur because one of the anticipated products is insoluble.
2) Example of gas formation reaction:
Carbonic acid
H2CO3 (aq) → H2O (l) + CO2 (g)
Sulfurous Acid
H2SO3 (aq) → H2O (l) + SO2 (g)
One of the products (AD or CB) in these reactions is in the gaseous form following the double replacement, such as hydrogen sulfide (H2S) or ammonia (NH3). Sulfuric acid or carbonic acid (H2CO3) might possibly be one of the byproducts (H2SO3). Both sulfurous acid and carbonic acid are unstable and will break down to produce gases called carbon dioxide and sulfur dioxide.
To learn more about Gases,
https://brainly.com/question/27870704
The correct question may be:
Write any two examples for a double replacement reaction.
Read the given equation.
2Na+ 2H₂O 2NaOH + H₂
During a laboratory experiment, a certain quantity of sodium metal reacted with water to produce sodium hydroxide and hydrogen gas. What was the initial quantity of
sodium metal used if 7.80 liters of H₂ gas were produced at STP?
07:29 grams
09.30 grams
12.2 grams
16.0 grams
Answers
Once alchol is in the bloodstrram it will reach the brain I'm a few
Answers
Once alcohol is in the bloodstream it will reach the brain in a few seconds to minutes, depending on various factors such as the amount and concentration of alcohol consumed, body weight, metabolism, and other individual factors.
Alcohol's Effects on BrainAlcohol can swiftly cross the blood-brain barrier after it is ingested, having an impact on the brain and neurological system. Depending on the quantity and frequency of drinking, alcohol's effects on the brain can range from minor disturbances in judgment and coordination to more serious consequences including loss of consciousness and, in the worst circumstances, death.
Long-term changes in brain structure and function, such as cognitive impairment and a higher chance of developing specific neurological and mental illnesses, can also result from chronic alcohol consumption.
Once alcohol is in the bloodstream it will reach the brain in a few seconds to minutes, depending on various factors such as the amount and concentration of alcohol consumed, body weight, metabolism, and other individual factors.
Learn more on effects of alcohol on the body here https://brainly.com/question/6511905
#SPJ1
In using the Haber process in the formation of ammonia, what mass of hydrogen is needed to produce 51.0 grams of ammonia? 3 H₂(g) + N2 (g) → 2 NH3(g).
Answers
The mass of hydrogen needed to produce 51.0 grams of ammonia is ≈ 9.07 grams.
To determine the mass of hydrogen required to produce 51.0 grams of ammonia (NH3) using the Haber process, we need to calculate the stoichiometric ratio between hydrogen and ammonia.
From the balanced chemical equation:
3 H₂(g) + N₂(g) → 2 NH₃(g)
We can see that for every 3 moles of hydrogen (H₂), we obtain 2 moles of ammonia (NH₃).
First, we need to convert the given mass of ammonia (51.0 grams) to moles. The molar mass of NH₃ is 17.03 g/mol.
Number of moles of NH₃ = Mass / Molar mass
= 51.0 g / 17.03 g/mol
≈ 2.995 moles
Next, using the stoichiometric ratio, we can calculate the moles of hydrogen required.
Moles of H₂ = (Moles of NH₃ × Coefficient of H₂) / Coefficient of NH₃
= (2.995 moles × 3) / 2
≈ 4.493 moles
Finally, we can convert the moles of hydrogen to mass using the molar mass of hydrogen (2.02 g/mol).
Mass of H₂ = Moles × Molar mass
= 4.493 moles × 2.02 g/mol
≈ 9.07 grams
Therefore, approximately 9.07 grams of hydrogen is needed to produce 51.0 grams of ammonia in the Haber process.
Know more about the mass of hydrogen here:
https://brainly.com/question/14083730
#SPJ8
A solution is prepared by dissolving 0.26 mol of hydrazoic acid and 0.26 mol of sodium azide in water sufficient to yield 1.00 L of solution. The addition of 0.05 mol of HCl to this buffer solution causes the pH to drop slightly. The pH does not decrease drastically because the HCl reacts with the ________ present in the buffer solution. The Ka of hydrazoic acid is 2.5 x 10^-5.
Answers
Answer:
The pH does not decrease drastically because the HCl reacts with the sodium azide (NaN₃) present in the buffer solution.
Explanation:
The buffer solution is formed by 0.26 moles of the weak acid, hydrazoic acid (HN₃), and by 0.26 moles of sodium azide (NaN₃). The equilibrium reaction of this buffer solution is the following:
HN₃(aq) + H₂O(l) ⇄ N₃⁻(aq) + H₃O⁺(aq)
The pH of this solution is:
\( pH = pka + log(\frac{[N_{3}^{-}]}{[HN_{3}]}) = -log(2.5 \cdot 10^{-5}) + log(\frac{0.26 mol/1 L}{0.26 mol/1 L}) = 4.60 \)
When 0.05 moles of HCl is added to the buffer solution, the following reaction takes place:
H₃O⁺(aq) + N₃⁻(aq) ⇄ HN₃(aq) + H₂O(l)
The number of moles of NaN₃ after the reaction with HCl is:
\( \eta_{NaN_{3}} = \eta_{i} - \eta_{HCl} = 0.26 moles - 0.05 moles = 0.21 moles \)
Now, the number of moles of HN₃ is:
\( \eta_{HN_{3}} = \eta_{i} + \eta_{HCl} = 0.26 moles + 0.05 moles = 0.31 moles \)
Then, the pH of the buffer solution after the addition of HCl is:
\( pH = pka + log(\frac{[N_{3}^{-}]}{[HN_{3}]}) = -log(2.5 \cdot 10^{-5}) + log(\frac{0.21 mol/V_{T}}{0.31 mol/V_{T}}) = 4.43 \)
The pH of the buffer solution does not decrease drastically, it is 4.60 before the addition of HCl and 4.43 after the addition of HCl.
Therefore, the pH does not decrease drastically because the HCl reacts with the sodium azide (NaN₃) present in the buffer solution.
I hope it helps you!
Please i meed help quick and thank you

Answers
It is the 4th scenario is the dependent event. There are 7 gold tokens and 4 silver tokens in a cup. The first student randomly draws a gold token and keeps it. A second student randomly draws a gold token from the cup.
How did we identify the dependent event?The fouth scenario is a dependent event because the probability of the second student drawing a gold token is affected by the outcome of the first student's draw.
If the first student draws a gold token, then there are only 6 gold tokens left in the cup, the probability changes. but if the first student does not draw a gold token, then there are 7 gold tokens left in the cup, the probability will remain the same
Find more exercises on dependent events;
https://brainly.com/question/11473170
#SPJ1
What physical property of matter can be measured using the triple beam balance?
A
volume
B
mass
C
height
D
length
SUBMIT ANSWER
Answers
Answer:
B) Mass- I searched it up
Can anyone help me understand how to calculate the moles of H+ and OH-?

Answers
To calculate the moles of H+ and OH-, you need to know the concentration of the solution in terms of its pH or pOH value.
How to calculate the molesWhen you get the pH of the solution, you can use this formula to calculate the concentration of H+ ions: [H+] = 10^(-pH)
Also, if you know the pOH of the solution, you can use this formula to calculate the concentration of OH- ions: [OH-] = 10^(-pOH)
Having determined the concentration of H+ and OH- ions, the molarity formula can be used to calculate the number of moles of each ion as follows: moles = concentration (in mol/L) x volume (in L)
Learn more about moles calculation here:
https://brainly.com/question/14357742
#SPJ1
What is the frequency of gamma radiation with energy of 6.96 x 10-14 J?
Answers
The frequency of gamma radiation is calculated to be = 1.11 *10^20 Hz.
What is gamma radiation?A gamma radiation is also known as gamma ray. It is a penetrating form of electromagnetic radiation arising from radioactive decay of atomic nuclei. It consists of shortest wavelength electromagnetic waves, even shorter than X-rays.
Gamma rays can pass through the human body completely and as they pass through, they cause ionizations that damage tissue and DNA.
As we know that E= hf
Hence, f= E/h
Given E= 6.96 x 10-14 J
And, Planck's constant, h = 6.626 *10^-34 m² kg/s
f = 6.96 * 10^-14J +/6.626 *10^-34
f = 1.11 *10^20 Hz
Hence, frequency of gamma radiation = 1.11 *10^20 Hz.
To know more about gamma radiation, refer
https://brainly.com/question/22166705
#SPJ1
what are the disadvantages of using coal as an energy source?; how did the steam engine improve manufacturing and transportation?; what uses did watt’s steam engine have during the industrial revolution?; explain how turnpikes and canals improved transportation during the industrial revolution?; how did the state of rail lines change during the period represented in the maps?
Answers
Air pollution is the major disadvantage of using coal as an energy source. The energy source for many machines and vehicles was steam power, which made it less expensive and simpler to create goods in huge quantities.
Watt continued to improve his ground-breaking design, making it possible for Boulton & Watt steam engines to operate machinery in paper, cotton, flour, and iron mills, textile factories, distilleries, canals, waterworks, and even an early steam train.
You could connect cities with canals and streamline and speed up inland transportation. The Erie Canal's construction was given the go-ahead by New York in April 1817.
The scientific advancements made during the Industrial Revolution in Great Britain and those made abroad when industrialization extended to other nations altered the path of human history and gave rise to the contemporary world we live in today.
For more information on coal energy kindly visit to
https://brainly.com/question/17443858
#SPJ4
Consider the reaction of 30.0 mL of 0.235 M BaI₂ with 20.0 mL of 0.315 M Na₃PO₄.
Which of the following compounds would be the precipitate that forms?
a) Bal2
b) Na3PO4
c) Ba3(PO4)2
d)Nal
Answers
Answer:
C
Explanation:
Because sodium is basically always soluble with any compound, it is between a and c. a is part of the reactant so it cant be A. So C.
The statement, that describes the compounds would be the precipitate that forms in the reaction is "Ba3(PO4)2"
What is precipitate?Precipitate is a solid generated by a change in a solution, usually due to a chemical reaction or a change in temperature that reduces a solid's solubility.
What is compound?The combination of more than one element will be identified ad compound.
When cations and anions in aqueous solution combine to create an insoluble ionic solid called a precipitate, double displacement reactions occur, resulting in the formation of a solid form residue. Except for salts of Group 1 metals and ammonium, salts of phosphates and carbonates ions are insoluble, according to the solubility flow chart. The production of a solid white precipitate is used to demonstrate the insoluble nature of barium phosphate.
Hence the correct option is c.
Learn more about precipitate here,
https://brainly.com/question/18109776
#SPJ2