Answers
Answer:
To find the percentage by volume of alcohol, we need to divide the volume of alcohol by the total volume of the solution, then multiply by 100%:
Volume of alcohol = 19 mL
Total volume of solution = 19 mL + 31 mL = 50 mL
Percentage by volume of alcohol = (19/50) x 100% = 38%
Therefore, the percentage by volume of alcohol in the solution is 38%.
Answer:
20% volume percentage I think
Related Questions
Which of the following represents the overall chemical equation for the reaction and the rate law for elementary step 2
O The overall reaction is H2(g) + 2ICI(g) -- > 2HCI(g) + I2(g) The rate law for step 2 is rate = k[HI][ICI]
O The overall reaction is H2(g) + 2ICI(g) -- > 2HCI(g) + I2(g) The rate law for step 2 is rate = k [H2] [HI] [ICI]
O The overall reaction is H2(g) + 2ICI(g) -- > 2HCI(g) + I2(g) The rate law for step 2 is rate = k [H2] [ICI]
Answers
The correct answer is Option 2: The overall reaction is>
H2(g) + 2ICI(g) -- > 2HCI(g) + I2(g)The reaction of hydrogen and iodine chloride produces hydrogen iodide as an intermediate. This means that the rate of the reaction is determined by the concentrations of all three species:
hydrogenhydrogen iodideiodine chloride.The rate law, which expresses the relationship between the rate of the reaction and the concentration of the reactants, is rate = k [H2] [HI] [ICI], where k is the rate constant. This means that the rate of the reaction is proportional to the concentrations of H2, HI, and ICI.
Learn more about reaction :
https://brainly.com/question/24795637
#SPJ4
Which of the following could be classified as unleavened bread?
sourdough bread
muffins
tortilla
bagels
Answers
Answer:
Tortilla
Explanation:
Answer:
tortilla
Explanation:
How many significant figures
are in this number?
23,479
Answers
how many moles of C8 H18 are present in a 546.23 gram sample?
Answers
Answer:
Given:
Compound C8H18
mass of compound- 546.23 gram
To find:
number of moles =?
Solution:
let's find out the molar mass of C8H18= 12×8+18×1 = 114 gram/mole
Number of moles= given mass/molar mass
=546.23/114
= 4.7818436487 moles.
Thanks for joining brainly community!
The Fischer esterification mechanism is examined in this question. The overall reaction is: Benzoic acid, C H 3 O H and H C l react to form a methyl ester, H 2 O and H C l. Benzoic acid is a carboxylic acid bonded to a benzene ring. Identify the results or mechanism of each step.
Answers
Answer:
See explanation and image attached
Explanation:
Fischer esterification is a type of reaction used to convert carboxylic acids to ester in the presence of excess alcohol and a strong acid which acts as a catalyst. Another final product formed in the reaction is water.
The mechanism for the fischer esterification of Benzoic acid and C H 3 O H in the presence of HCl as the catalyst is shown in the image attached to this answer.
The final products of the reaction are methyl benzoate, water and H^+ as shown in the image attached.

The methyl ester, water, and the acid catalyst (HCl) are byproducts of the Fischer esterification process, which involves protonation, nucleophilic attack, elimination, and deprotonation processes.
Carbonyl oxygen protonation: The carbonyl oxygen of the carboxylic acid (benzoic acid) is protonated by the acid catalyst (HCl) in the first step. The protonation of the carbonyl carbon increases its electrophilicity and promotes the alcohol's nucleophilic assault. Attack by the alcohol's nucleophilic oxygen (methanol, CH3OH) on the protonated carboxylic acid's carbonyl carbon results in the formation of a tetrahedral intermediate. The acid catalyst also helps with this phase. Elimination of water: In the following step, the water molecule must be removed from the tetrahedral intermediate. The hydroxyl group (-OH) from the carboxylic acid and a hydrogen from the hydroxyl group of the alcohol are removed to create this water molecule. Deprotonation: A deprotonation occurs after the removal of water.
To know more about Fischer esterification process, here:
https://brainly.com/question/31041190
#SPJ6
If the volume is 10 and the mass of water is 9.99 what is the density
Answers
Answer:
0.999
Explanation: divide by 10
An organic molecule must contain carbon and nitrogen. True or false
Answers
Answer: False
Explanation:
Except for a few substances like carbonates, carbides, cyanides, and cyanates, every molecule that includes carbon and hydrogen is considered to be organic. A molecule need not include nitrogen to be regarded as organic.
Amino acids, proteins, and nucleic acids are examples of organic molecules that include both carbon and nitrogen. However, not all organic substances contain nitrogen. The most prevalent organic substances are hydrocarbons and their derivatives, which are made up entirely of hydrogen and carbon.
It is essential to remember that the term "organic" is used in various settings and denotes various things depending on the industry.
While "organic" refers to items produced without the use of synthetic pesticides, fertilizers, or other chemicals in agriculture and food science, the term "organic" is used to designate substances that include carbon and hydrogen in chemistry.
Question 2
The higher the energy, the higher or lower the wavelength? This is an example of a/an direct or inverse relationship?
Answers
Answer:
the higher the energy tje lower the wavelength
Explanation:
inverse relationship
Answer:
It's an example of an inverse relationship :)
7. What is the volume of the
composite
solid?
4 in.
3 in.
3 in.

Answers
Answer:
The volume of Component 1 is 36 cubic inches.
Explanation:
To calculate the volume of a composite solid, we need to determine the individual volumes of the different components and then add them together.
In this case, the composite solid consists of multiple components with the following dimensions:
Component 1:
Length: 4 inches
Width: 3 inches
Height: 3 inches
To find the volume of Component 1, we multiply the length, width, and height together:
Volume of Component 1 = Length x Width x Height = 4 in x 3 in x 3 in = 36 cubic inches
Therefore, the volume of Component 1 is 36 cubic inches.
Please provide the dimensions of the remaining components of the composite solid, and I will calculate the total volume by summing up the individual volumes.
Write a Lewis structure for the phosphorus trifluoride molecule, PF3. Draw the Lewis dot structure for PF3. Draw the molecule by placing atoms on the grid and connecting them with bonds. Include all lone pairs of electrons.
Answers
Answer:
Explanation:
Hello there!
In this case, according to the given information, it turns out possible for us to draw this Lewis dot structure by firstly realizing P has five valence electrons and F has seven because they are in groups VA and VIIA respectively, which means that the central atom is P with three F atoms around it as shown on the attached.
Also notice each fluorine atom has three lone pairs as their atoms just need one bond to complete the octet and the P atom has one lone pair as it needs three bonds to complete it.
Regards!

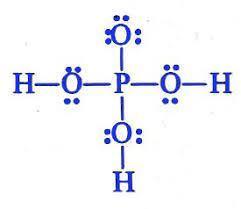
Which is the Lewis structure for H3PO4? An upper P is single bonded above to an O, and to the left, right, and below to an O single bonded to an H. The O above the P has three pairs of dots to the left, above, and below; the O's to the sides have pairs of dots above and below, and the O below the P has pairs of dots right and left. A central upper P is single bonded left, right, above, and below to upper Os. The O above the P is single bonded to upper H on the left and the right, and has two electron dots above it. The O below the P is single bonded to an H below, and has pairs of electron dots to the left and right. A central upper P is double bonded to an O above, and single-bonded to an upper O single-bonded to an upper H to the left and the right. The O above the P has three pairs of electron dots, to the left, above, and to the right; the O's to the right and left have pairs of dots above and below. A central upper P is bonded to an upper H above, an upper O below, and upper O's bonded to upper H's to the left and the right. The O below the P has three pairs of electron dots, to the left, right, and below; the other two O's have pairs of dots above and below. A central upper P is double bonded to an O above, and single-bonded to an upper O single-bonded to an upper H to the left and the right. The O above the P has three pairs of electron dots, to the left, above, and to the right; the O's to the right and left have pairs of dots above and below.
Answers
Answer:
It is A.
Explanation:
I took the test.
The Lewis structure shows the arrangement of valence electrons in H3PO4.
The Lewis structure gives us a picture of the number of valence electrons in a molecule. This is because, in a Lewis structure, the electrons in the molecule are shown as dots. A single line may be used to show shared electrons in a covalent bond.
The correct Lewis structure of H3PO4 is an upper P is single bonded above to an O, and to the left, right, and below to an O single bonded to an H. The O above the P has three pairs of dots to the left, above, and below; the O's to the sides have pairs of dots above and below, and the O below the P has pairs of dots right and left.
Learn more: https://brainly.com/question/4144781
Combined Gas Law equation is represented by
A)T1 / P1V1 = T2 / P2V2
B) P1V1 / T1 = P2V2 / T2
C)P1V1 / T1 = (P2V2 / T2)2
D)PV = nRT
Answers
The combined Gas Law equation is represented by B) P1V1 / T1 = P2V2 / T2.
The combined gas law is the law that combines Charles’s law, Gay-Lussac’s law, and Boyle’s law.
Combined gas law can be mathematically expressed as
k = PV/T
Where,
P = pressure
T = temperature in kelvin
V = volume
K = constant (units of energy divided by temperature)
When two substances are compared in two different conditions, the law can be stated as,
P1V1/T1 = P2V2/T2
Where,
P1 = initial pressure
V1 = initial volume
T1 = initial temperature
P2 = final pressure
V2= final volume
T2 = final temperature
To learn more about combined Gas Law,
https://brainly.com/question/13154969
Problem 1. What masses of 15% and 20% solutions are needed to prepare 200 g of 17% solution?
Problem 2. What masses of 18% and 5% solutions are needed to prepare 300 g of 7% solution?
Problem 3. 200 g of 15% and 350 g of 20% solutions were mixed. Calculate mass percentage of final solution.
Problem 4. 300 g of 15% solution and 35 g of solute were mixed. Calculate mass percentage of final solution.
Problem 5. 400 g of 25% solution and 150 g of water were mixed. Calculate mass percentage of final solution.
Answers
Problem 1:
we need 80 g of the 15% solution and 120 g of the 20% solution.
Let x be the mass of the 15% solution needed and y be the mass of the 20% solution needed.
x + y = 200 (total mass of the two solutions)
0.15x + 0.2y = 0.17(200) (total amount of solute in the two solutions)
Solving these equations, x = 80 g and y = 120 g.
Therefore, we need 80 g of the 15% solution and 120 g of the 20% solution.
Problem 2:
we need 120 g of the 18% solution and 180 g of the 5% solution.
Let x be the mass of the 18% solution needed and y be the mass of the 5% solution needed.
x + y = 300
0.18x + 0.05y = 0.07(300)
Solving these equations, x = 120 g and y = 180 g.
Therefore, we need 120 g of the 18% solution and 180 g of the 5% solution.
Problem 3:
The mass percentage of the final solution is 135 g/550 g × 100% = 24.55%.
The total mass of the final solution is 200 g + 350 g = 550 g.
The total amount of solute in the final solution is:
0.15(200 g) + 0.20(350 g) = 65 g + 70 g = 135 g.
Therefore, the mass percentage of the final solution is 135 g/550 g × 100% = 24.55%.
Problem 4:
The mass percentage of the final solution is 110 g/335 g × 100% = 32.84%.
The total mass of the final solution is 300 g + 35 g = 335 g.
The total amount of solute in the final solution is:
0.15(300 g) + 35 g = 75 g + 35 g = 110 g.
Therefore, the mass percentage of the final solution is 110 g/335 g × 100% = 32.84%.
Problem 5:
The mass percentage of the final solution is 18.18%.
Calculate the final mass of the solution:
Final mass = 400 g + 150 g = 550 g
Calculate the mass of solute in the 25% solution:
Mass of solute = 0.25 x 400 g = 100 g
Calculate the mass percentage of the final solution:
Mass percentage = (mass of solute ÷ final mass) x 100%
Mass percentage = (100 g ÷ 550 g) x 100%
Mass percentage = 18.18%
To know more about mass of solutions, visit:
https://brainly.com/question/29482678
#SPJ1
The enthalpy of vaporization for methanol is 35.2 kJ/mol. Methanol has a vapor pressure of 1 atm at 64.7 oC. Using the Clausius-Clapeyron equation, what is the vapor pressure for methanol at 55.5 oC? Give your answer in atmospheres, to the third decimal point.
Answers
Answer: 55.5 oC is 0.014 atm (3rd decimal point)
Explanation:
The Clausius-Clapeyron equation is given as:
ln(P2/P1) = -(ΔH_vap/R) * (1/T2 - 1/T1)
where:
P1 = vapor pressure at temperature T1
P2 = vapor pressure at temperature T2
ΔH_vap = enthalpy of vaporization
R = gas constant = 8.314 J/(mol*K)
Converting the enthalpy of vaporization to J/mol:
ΔH_vap = 35.2 kJ/mol = 35,200 J/mol
Converting temperatures to Kelvin:
T1 = 64.7 + 273.15 = 337.85 K
T2 = 55.5 + 273.15 = 328.65 K
Substituting the values into the equation and solving for P2:
ln(P2/1 atm) = -(35,200 J/mol / 8.314 J/(mol*K)) * (1/328.65 K - 1/337.85 K)
ln(P2/1 atm) = -4.231
P2/1 atm = e^(-4.231)
P2 = 0.014 atm
Therefore, the vapor pressure for methanol at 55.5 oC is 0.014 atm, to the third decimal point.
A 0.500 g impure sample of LiNO3 is heated, causing it to decompose according to the following equation: 4LINO3(s) — Li,O(s) + 4NO2(g) + O2(g) The gases produced are collected over water at 27°C and 1.00 atm external pressure, and occupy a volume of 55.2 mL. Calculate the partial pressure of O, in the mixture? What is the percentage of LINO3 in the sample? The partial pressure of water is 26.7 mm Hg at 27°C.
Answers
The partial pressure of O2 and the percentage of LiNO3 in an impure sample after undergoing a decomposition reaction can be determined through the following steps:
Find the number of moles of gas produced: The volume of the gas mixture and its temperature are used to find the number of moles of gas using the ideal gas law.
Determine the partial pressure of O2: The number of moles of O2 produced is used in the ideal gas law to find its partial pressure.
Find the total pressure: The partial pressures of O2, NO2, and water are added to find the total pressure of the gas mixture.
Determine the percentage of LiNO3: The mass of LiNO3 in the original sample is calculated based on the number of moles of gas produced and its molar mass, and then divided by the total mass of the original sample to find the percentage of LiNO3.
The final answer is the partial pressure of O2 is 0.452 atm and the percentage of LiNO3 in the original sample is 43.5%.
To learn more about partial pressure please click on below link
https://brainly.com/question/15075781
#SPJ4
How are we able to see cells?
Answers
Answer:
Contemporary light microscopes are able to magnify objects up to about a thousand times. Since most cells are between 1 and 100 μm in diameter, they can be observed by light microscopy, as can some of the larger subcellular organelles, such as nuclei, chloroplasts, and mitochondria.
Draw the structure of phosphatidylserine and discuss its components
Answers
Phosphatidylserine is a type of phospholipid that is mainly found in cell membranes. Its structure is made up of two fatty acid chains, a phosphate group, a serine molecule, and a glycerol molecule.
The fatty acid chains are hydrophobic, meaning they repel water, while the phosphate group and serine molecule are hydrophilic, meaning they attract water.
The glycerol molecule acts as a bridge that connects the two fatty acid chains to the phosphate group and serine molecule.
The structure of phosphatidylserine is important for its function in the cell membrane.
Because of the hydrophobic and hydrophilic components of its structure, phosphatidylserine is able to form a lipid bilayer, which is a barrier that separates the inside of the cell from the outside environment.
The hydrophilic heads of the phosphatidylserine molecules face outward and interact with water, while the hydrophobic tails face inward and repel water.
Phosphatidylserine also plays a role in cell signaling and apoptosis, which is programmed cell death.
It acts as a signaling molecule by binding to proteins that are involved in cellular pathways.
In addition, phosphatidylserine is translocated to the outer leaflet of the cell membrane during apoptosis, which signals to immune cells that the cell is ready to be removed.
In conclusion, the structure of phosphatidylserine is made up of two fatty acid chains, a phosphate group, a serine molecule, and a glycerol molecule. Its hydrophobic and hydrophilic components allow it to form a lipid bilayer in cell membranes, and it also plays a role in cell signaling and apoptosis.
For more such questions on Phosphatidylserine
https://brainly.com/question/16179573
#SPJ8
Bromine (Br) has ____ valence electrons. When it forms an ionic bond, it _____ one electron and has a charge of _____
Answers
Answer:
7 valence e-, obtain e-, negative
PLS HELP! WILL GIVE 30 POINTS!!!
I have 0,0004 gramms of CO2, how many grams of C are there?
Answers
Answer:
0.00011 [gr].
Explanation:
1. one mol of CO2 weights 12+32=44 [gr];
2. part of C in CO2 is 12/44=3/11, then
3. the required mass of C in 0.0004 [gr] is 0.0004*3/11≈0.0001(09) [gr].
A 54.2 g sample of polystyrene, which has a specific heat capacity of 1.880 J-gc, is put into a calorimeter (see sketch at
right) that contains 100.0 g of water. The temperature of the water starts off at 21.0 °C. When the temperature of the water stops
changing it's 34.3 °C. The pressure remains constant at 1 atm.
Calculate the initial temperature of the polystyrene sample. Be sure your answer is rounded to the correct number of significant
digits.
thermometer.
insulated
container
water
sample.
a calorimeter
Answers
Tthe initial temperature of the polystyrene sample is 39.4°C.
Given: Mass of polystyrene sample = 54.2 gSpecific heat of polystyrene = 1.880 J-g°CWater mass = 100.0 g Initial water temperature = 21.0°CWater final temperature = 34.3°CPressure remains constant at 1 atmFormula used:Heat gained by water = heat lost by polystyreneHence,Heat lost by polystyrene = Heat gained by water=> mcΔT = mcΔTwhere,m = mass of polystyrene or waterc = specific heat capacityΔT = change in temperatureThe temperature change is ΔT = 34.3°C - 21.0°C = 13.3°CNow we can use this temperature change to calculate the initial temperature of the polystyrene.Taking the water's specific heat capacity, c = 4.184 J/g°CHeat gained by water = (100.0 g)(4.184 J/g°C)(13.3°C) = 5574 JHeat lost by polystyrene = 5574 JTaking the polystyrene's specific heat capacity, c = 1.880 J/g° ) = 13.3°C Now let's calculate the mass of polystyrene using the specific heat capacity formula.5574 J = (54.2 g)(1.880 J/g°C)(13.3°C - Ti)Ti = 39.4°C
for more questions on polystyrene
https://brainly.com/question/15913091
#SPJ8
The pOH of a solution is 6.0. Which statement is correct?
Use pOH = -log[OH-] and PH+pOH = 14.
The pH of the solution is 20.0.
O The concentration of OH ions is 1.0 x 108 M.
The concentration of OH ions is 1.0 x 106 M.
O The pH of the solution is 8.0.
A
Answers
At pOH value of 6.0 the pH value of the following solution is 8.0 and the concentration of [\(H^{+}\) ] ion is \(10^{-8}\)
In this question we will apply the formula
pH +pOH = 14 . . . . . . . . . . . . .(1)
where pH = concentration of [\(H^{+}\) ] ion
pOH = concentration of [\(OH^{-}\) ] ion
As per the question
pOH =6.0
Putting the value of pOH in equation (1) we get the value of pH
pH + 6.0 =14
pH = 14 -6.0
pH = 8.0
The value of pH if the pOH value is 6.0 is 8.0
To find the concentration of \(H^{+}\) ion we will use the following formula
This is calculated by the formula
[\(H^{+}\)} = \(10^{-pH}\)
where we will write the values of pH
Hence the concentration of [\(H^{+}\)} ion is \(10^{-8}\)
Therefore at pOH of 6.0 the pH value of the following solution is 8.0 and the concentration of [\(H^{+}\) ] ion is \(10^{-8}\)
Read more about pH
https://brainly.com/question/11300720
The complete question is -
What is the pH value and concentration of [\(H^{+}\) ] ion of the following if the pOH value of the solution is 6.0 ?
Question 10 (1 point)
Match the prefixes to their long form.
Column A
Column B
1.
1 kilometer (km)
1000 meters
millimeter (mm)
b. meters
. 3.
centimeters (cm)
c. 0.001 meters
4.
base unit
d. 0.01 meters

Answers
Answer:
A,C,D,B
Explanation:
1killometer=1000m
1mm=0.001m
1cm=0.01m
base unit of length is meter
Answer:1 kilometer 1,000 meters
1 centimeter 10 millimeters
1 meter 100 centimeters
Explanation:
The isotope of an atom containing 31 protons and 39 neutrons suddenly has two neutrons added to it. What isotope is create?
Answers
what was the name of yesterday's supermoon
Answers
Answer: May's full moon or Flower moon
Explanation:
Plants go through seasonal changes from summer to fall because temperatures ___________. A: begin to cool daylight hours decrease.
B: begin to cool and daylight hours in increase.
C: warm up in daylight hours decrease.
Or D: warm up, and daylight hours increase.
30 POINTS IF YOU ANSWER THIS QUESTION ALSO, I HAVE 15 MINS
Also, there’s no science so I picked “ chemistry”
Answers
A: begin to cool and daylight hours decrease.
Plants go through seasonal changes from summer to fall because of the changes in temperature and the length of daylight hours. As summer ends and fall begins, temperatures begin to cool down and the days become shorter. This change in temperature and daylight hours triggers physiological changes in plants, such as the slowing down of growth and the production of pigments like anthocyanins, which give leaves their characteristic red and orange colors in the fall. These changes allow the plant to prepare for the colder winter months and conserve energy for the upcoming spring growth season.
Answer:
a. begin to cool and daylight hours decrease
hope this helps ;)
Compare and contrast the following types of chemical reactions (5 points each): a. Combination b. Decomposition c. Replacement
It needs to be in two to three paragraphs.
Answers
Answer:
Combination reactions, also known as synthesis reactions, involve the combination of two or more reactants to form a single product. These reactions often involve the formation of chemical bonds between the reactants, and they are usually exothermic, meaning that they release energy in the form of heat. Examples of combination reactions include the formation of water from hydrogen and oxygen, the synthesis of ammonia from nitrogen and hydrogen, and the combination of sodium and chlorine to form sodium chloride.
Decomposition reactions involve the breakdown of a single compound into two or more simpler substances. These reactions often involve the breaking of chemical bonds, and they are usually endothermic, meaning that they absorb energy from their surroundings. Examples of decomposition reactions include the breakdown of water into hydrogen and oxygen by electrolysis, the decomposition of hydrogen peroxide into water and oxygen, and the thermal decomposition of calcium carbonate into calcium oxide and carbon dioxide.
Replacement reactions, also known as substitution reactions, involve the replacement of one element or group of atoms in a compound by another element or group. These reactions often involve the transfer of electrons between reactants, and they can be either exothermic or endothermic depending on the specific reaction. Examples of replacement reactions include the displacement of hydrogen by a metal in a metal-acid reaction, the substitution of chlorine for hydrogen in an alkyl halide, and the replacement of a metal ion by another metal ion in a precipitation reaction.
Overall, combination reactions involve the combination of two or more reactants to form a single product, decomposition reactions involve the breakdown
does a negative exponent mean that the number is less than 1
Answers
From go ogle
The reaction between water and ammonium nitrate results in_____.
A. Light is emitted.
B. A precipitate (solid) is formed.
C. A temperature increase.
D. A temperature decrease.
Answers
The reaction between water and ammonium nitrate results in increase in a temperature.
Hence, Option C is the correct answer.
When ammonium nitrate is dipped in a beaker which contains the water, the beaker becomes cold because it is an endothermic reaction. The endothermic reaction is a reaction that it absorbs energy from the outside in the form of heat.
Thus we can say that hydrating ammonium nitrate is an endothermic reaction, because of that the beaker becomes cold.
We feels cold when ammonium nitrate is mixed in water that shows an endothermic reaction. In an endothermic reaction, the ammonium nitrate mixed in water, a chemical reaction occurs which absorbs heat rather than releases it.
Learn more about the Endothermic reaction here: https://brainly.com/question/28610140
Which state of category of elements has the greatest number of elements? Least?
Answers
semiconductors- least
Hurricanes and tropical storms gain their power from heated water evaporating from the ocean. Write a paragraph explaining why hurricanes typically weaken as they move into areas of cool water or over land.
Answers
As the disturbance gets to the land the warm ocean waters are no longer around thus the hurricane would die off rapidly
What is hurricane?The hurricane tends to occur when there is a rise of the warm air over the ocean. It should be noted that in the event of the hurricane there would be a sudden rising of the sea and this is going to rise so high that the violent waves would now begin to invade the land and cause a lot of destruction on its path as it is moving out from the ocean where the disturbance started.
Hence we can see that the origin of the hurricane is from some sort of a convection current that is taking place in the ocean and the hurricane must also be sustained by the existence of this convection current as the disturbance is moving away from the sea.
Learn more about Hurricane:https://brainly.com/question/13661501
#SPJ1