Answers
Answer:
the substance is an acid
Related Questions
Milk of magnesia, which is an aqueous suspension of magnesium hydroxide, is used as an antacid in the reaction below. How many molecules of HCl would have to be present to form 34.52 g of MgCl₂?
Mg(OH)₂(s) + 2 HCl(aq) → 2 H₂O(l) + MgCl₂(aq)
Answers
Approximately 4.37 x 10^23 molecules of HCl would be required to form 34.52 g of MgCl₂.
To determine the number of molecules of HCl required to form 34.52 g of MgCl₂, we need to use the molar mass and stoichiometry of the balanced equation:
Mg(OH)₂(s) + 2 HCl(aq) → 2 H₂O(l) + MgCl₂(aq)
The molar mass of MgCl₂ is 95.21 g/mol.
First, we need to calculate the number of moles of MgCl₂ formed:
Moles of MgCl₂ = mass of MgCl₂ / molar mass of MgCl₂
Moles of MgCl₂ = 34.52 g / 95.21 g/mol
Moles of MgCl₂ = 0.363 mol
According to the balanced equation, the stoichiometric ratio between HCl and MgCl₂ is 2:1. Therefore, the moles of HCl required can be calculated as follows:
Moles of HCl = 2 * Moles of MgCl₂
Moles of HCl = 2 * 0.363 mol
Moles of HCl = 0.726 mol
To calculate the number of molecules, we need to use Avogadro's number, which is approximately 6.022 x 10^23 molecules/mol.
Number of molecules of HCl = Moles of HCl * Avogadro's number
Number of molecules of HCl = 0.726 mol * 6.022 x 10^23 molecules/mol
Number of molecules of HCl = 4.37 x 10^23 molecules
Therefore, approximately 4.37 x 10^23 molecules of HCl would be required to form 34.52 g of MgCl₂.
For more such questions on molecules
https://brainly.com/question/1351818
#SPJ8
The pOH of a solution is 6.0. Which statement is correct?
Use pOH = -log[OH-] and PH+pOH = 14.
The pH of the solution is 20.0.
O The concentration of OH ions is 1.0 x 108 M.
The concentration of OH ions is 1.0 x 106 M.
O The pH of the solution is 8.0.
A
Answers
At pOH value of 6.0 the pH value of the following solution is 8.0 and the concentration of [\(H^{+}\) ] ion is \(10^{-8}\)
In this question we will apply the formula
pH +pOH = 14 . . . . . . . . . . . . .(1)
where pH = concentration of [\(H^{+}\) ] ion
pOH = concentration of [\(OH^{-}\) ] ion
As per the question
pOH =6.0
Putting the value of pOH in equation (1) we get the value of pH
pH + 6.0 =14
pH = 14 -6.0
pH = 8.0
The value of pH if the pOH value is 6.0 is 8.0
To find the concentration of \(H^{+}\) ion we will use the following formula
This is calculated by the formula
[\(H^{+}\)} = \(10^{-pH}\)
where we will write the values of pH
Hence the concentration of [\(H^{+}\)} ion is \(10^{-8}\)
Therefore at pOH of 6.0 the pH value of the following solution is 8.0 and the concentration of [\(H^{+}\) ] ion is \(10^{-8}\)
Read more about pH
https://brainly.com/question/11300720
The complete question is -
What is the pH value and concentration of [\(H^{+}\) ] ion of the following if the pOH value of the solution is 6.0 ?
Somebody help me asap please giving brainliest

Answers
Answer:
fire > rocks > air
PLEASE HELP QUICKK
Calculate the energy of combustion for one mole of butane if burning a 0.367 g sample of butane (C4H10) has increased the temperature of a bomb calorimeter by 7.73 °C. The heat capacity of the bomb calorimeter is 2.36 kJ/ °C.
Answers
The energy of combustion for one mole of butane to be approximately 2888.81 kJ/mol.
To calculate the energy of combustion for one mole of butane (C4H10), we need to use the information provided and apply the principle of calorimetry.
First, we need to convert the mass of the butane sample from grams to moles. The molar mass of butane (C4H10) can be calculated as follows:
C: 12.01 g/mol
H: 1.01 g/mol
Molar mass of C4H10 = (12.01 * 4) + (1.01 * 10) = 58.12 g/mol
Next, we calculate the moles of butane in the sample:
moles of butane = mass of butane sample / molar mass of butane
moles of butane = 0.367 g / 58.12 g/mol ≈ 0.00631 mol
Now, we can calculate the heat released by the combustion of the butane sample using the equation:
q = C * ΔT
where q is the heat released, C is the heat capacity of the calorimeter, and ΔT is the change in temperature.
Given that the heat capacity of the bomb calorimeter is 2.36 kJ/°C and the change in temperature is 7.73 °C, we can substitute these values into the equation:
q = (2.36 kJ/°C) * 7.73 °C = 18.2078 kJ
Since the heat released by the combustion of the butane sample is equal to the heat absorbed by the calorimeter, we can equate this value to the energy of combustion for one mole of butane.
Energy of combustion for one mole of butane = q / moles of butane
Energy of combustion for one mole of butane = 18.2078 kJ / 0.00631 mol ≈ 2888.81 kJ/mol
Therefore, the energy of combustion for one mole of butane is approximately 2888.81 kJ/mol.
In conclusion, by applying the principles of calorimetry and using the given data, we have calculated the energy of combustion for one mole of butane to be approximately 2888.81 kJ/mol.
For more questions on molar mass, click on:
https://brainly.com/question/837939
#SPJ8
Suppose a solution has a density of 1.87 g/mL. If a sample has a mass of 17.5 g the volume of the sample in mL is what?
Answers
We can use the formula:
Density = Mass/Volume
Rearranging the formula gives:
Volume = Mass/Density
Substituting the given values gives:
Volume = 17.5 g / 1.87 g/mL = 9.36 mL.
Matter includes all of the following EXCEPT-
Osmoke
о
water vapor
light
air
Answers
Answer: Light is not a matter.
Explanation:
Answer:light
Explanation:
A gas has a volume of 50.0 mL at a temperature of 10.0 K and a pressure of 760. kPa. What will be the new volume when the temperature is changed to 20.0 K and the pressure is changed to 380. kPa?
Answers
To solve this problem using the gas laws, we need to use the Ideal Gas Law. This law states that the product of the pressure and the volume of a gas is proportional to the absolute temperature.
The equation of the Ideal Gas Law is the following:
\(\boxed{\large\displaystyle\text{$\begin{gathered}\sf \bf{\dfrac{P_1V_1}{T_1}=\frac{P_2V_2}{T_2} } \end{gathered}$} }\)
Where:
P₁ = initial pressure = 760 kPaV₁ = initial volume = 50.0 mL = 0.050 LT₁ = initial temperature = 10.0 KP₂ = Final pressure = 380 kPaT₂ = final temperature = 20.0 KV₂ = Final volume = ?We clear for V₂:
\(\boxed{\large\displaystyle\text{$\begin{gathered}\sf \bf{V_2=\frac{P_1V_1T_2}{P_2T_1 } } \end{gathered}$} }\)
Where:
P₁ = initial pressure V₁ = initial volumeT₁ = initial temperatureP₂ = Final pressureT₂ = final temperatureV₂ = Final volumeSubstituting the known values:
\(\boxed{\large\displaystyle\text{$\begin{gathered}\sf \bf{V_2=\frac{760\not{kPa}\times0.050 \ L\times20.0\not{k} }{ 380\not{kPa}\times10.0\not{k} } } \end{gathered}$} }\)
\(\boxed{\large\displaystyle\text{$\begin{gathered}\sf \bf{V_2=\frac{760 \ L}{3800 } } \end{gathered}$} }\)
\(\boxed{\boxed{\large\displaystyle\text{$\begin{gathered}\sf \bf{V_2\approx0.2 \ Liters} \end{gathered}$} }}\)
When the temperature changes to 20.0 K and the pressure changes to 380 kPa, the new volume will be approximately 0.2 L (200.0 mL).The question is in the picture

Answers
Charles's law of gases states that the density of an ideal gas is inversely proportional to its temperature at constant pressure.
The equation is as follows;
Va/Ta = Vb/Tb
Where;
Va and Ta = initial volume and temperature respectivelyVb and Tb = final volume and temperature respectively0.67/362 = 1.12/Tb
0.00185Tb = 1.12
Tb = 605.41K
This temperature in °C is 605.41 - 273 = 332°C
Learn more about Charles's law at: https://brainly.com/question/16927784
#SPJ1
Identify the activated complex in the following reaction.
a. CuFeSO
b. FeFe
c. FeCuSO4
d. FeSO4
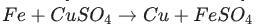
Answers
The activated complex in the following reaction is: FeCuSO4. The activated complex is a transition state that is an intermediate structure in a chemical reaction. Option C)
An activated complex is a structure that exists temporarily during a chemical reaction and corresponds to the top of the energy barrier that must be overcome for the reaction to proceed to completion.
The activated complex in the following reaction is: FeCuSO4. The activated complex is a transition state that is an intermediate structure in a chemical reaction. It is the structure with the greatest energy within the reaction process and is used to determine the rate at which the reaction occurs. An activated complex exists when the energy required to break the old bonds and form new ones has been absorbed. It has a specific configuration and energy content that is precisely defined.
A chemical reaction is the process by which atoms or groups of atoms in molecules interact to form new molecules. A chemical reaction is caused by the motion of electrons, which are negatively charged particles that surround atomic nuclei. The reaction proceeds through the formation of an intermediate species known as the transition state or activated complex. Reaction mechanisms are the sequence of steps involved in a chemical reaction. These steps describe the intermediate species formed as the reactants are converted to products. Hence option C) is correct.
for more questions on reaction
https://brainly.com/question/25769000
#SPJ8
Calculate the gram molecular masses of the following species and label those that are compounds: H2O (water), C12H22O11 (table sugar), ---(C3H6)1000--- (polypropylene plastic), S6 (sulfur powder), Al2O3 (aluminum oxide), CHCl3 (chloroform).
Answers
The gram molecular masses of the following species are
H₂O is a compound.
molecular mass H₂O :
mass of hydrogen = 1 g/mol
mass of oxygen = 16 g/mol
molecular mass of H₂O = 2 × 1 + 16
= 18 g/mol
C₁₂H₂₂O₁₁ is a compound.
mass of carbon = 12 g/mol
mass of hydrogen = 1 g/mol
mass of oxygen = 16 g/mol
molecular mass of C₁₂H₂₂O₁₁ = 12 × 12 + 22 × 1 + 11 × 16
= 144 + 22 + 176
= 342 g/mol
-----(C₃H₆)₁₀₀----- Polypropylene is a compound.
mass of carbon = 12 g/mol
mass of hydrogen = 1 g/mol
molecular mass of -----(C₃H₆)₁₀₀₀----- = (3 × 12 + 6 × 1 ) × 1000
= 42000 g/mol
Al₂O₃ ( aluminum oxide ) is chemical compound.
mass of aluminum = 27 g/mol
mass of oxygen = 16 g/mol
molecular mass = 2 × 27 + 3 × 16
= 102 g/mol
S₆ (sulfur powder)
mass of sulfur = 32 g/mol
molar mass of = 6 × 32
= 192 g/mol
CHCl₃ ( chloroform) is a compound
mass of carbon = 12 g/mol
mass of hydrogen = 1 g/mol
mass of chlorine = 35 g/mol
molar mass of CHCl₃ = 12 + 1 + 3 × 35
= 119 g/mol
To learn more about molecular mass here
https://brainly.com/question/11984639
#SPJ1
What mass of NaCl is needed to produce a 26.4 mol/L with a 1.7 L volume?
Answers
we would need 2625.13 grams (or 2.62513 kilograms) of NaCl.
To calculate the mass of NaCl required to produce a 26.4 mol/L solution with a 1.7 L volume, we need to use the formula that relates the mass of solute, moles of solute, and molarity:Molarity (M) = moles of solute / liters of solution Rearranging this formula, we get:moles of solute = Molarity (M) x liters of solutionWe can use this formula to find the moles of NaCl needed:moles of NaCl = 26.4 mol/L x 1.7 L = 44.88 molNow, we can use the molar mass of NaCl to convert from moles to grams. The molar mass of NaCl is 58.44 g/mol:mass of NaCl = moles of NaCl x molar mass of NaClmass of NaCl = 44.88 mol x 58.44 g/mol = 2625.13 gTo produce a 26.4 mol/L solution with a 1.7 L volume.
for more question on NaCl
https://brainly.com/question/23269908
#SPJ8
Jonathon is conducting an experiment to determine how much precipitate (solid product) will form when combining measured volumes of Aich, and NaOH. According to his calculations the reaction should produce 26.0 grams of solid AKOH), when combined. However, when Jonathon measures the mass of the solid precipitate formed in his experiment, he finds that the experiment actually produced 24.5 grams of Al(OH).
Answers
Jonathon's experiment produced 24.5 grams of Al(OH), which is less than the predicted amount of 26.0 grams of AKOH. The discrepancy could be due to measurement errors, incomplete reaction.
What is discrepancy?
To determine the cause of the discrepancy, Jonathon should first review his experimental procedure and make sure that all measurements and calculations were performed accurately. He should also check that the reactants were mixed thoroughly and that the reaction was allowed to proceed to completion. If any errors or inconsistencies are identified, Jonathon should correct them and repeat the experiment to obtain more accurate results.
If the experimental procedure was carried out correctly and the discrepancy cannot be attributed to measurement errors, Jonathon should consider the possibility of impurities in the reactants. Even small amounts of impurities can affect the outcome of a chemical reaction, so it is important to use high-quality, pure chemicals in experiments whenever possible.
Overall, the most important thing for Jonathon to do in this situation is to carefully review his experimental data and methodology, and to identify any potential sources of error or uncertainty. By doing so, he can improve the accuracy and reliability of his results and draw more meaningful conclusions from his experiment.
To know more about reactants, visit:
https://brainly.com/question/13005466
#SPJ9
Why are the united nation members upset with Wakanda?
Answers
The "Wakanda speech," six weeks have passed, and the world is still in shock.
Thus, Global leaders and analysts were taken aback by King T'Challa's declaration at the United Nations General Assembly that the Kingdom of Wakanda is not a developing country of textiles, farms, and shepherds with a GDP per person of roughly $760 but rather a technological superpower and Wakanda speech.
The country's widespread employment of cutting-edge magnetic levitation trains, flying machines, opaque holograms, and spinal cord-healing beads has led to the coining of the phrase "uber-developed" nation.
The most watched video ever is currently "Welcome to the Future," an introduction video created by Wakanda's recently established Ministry of Foreign Affairs.
Thus, The "Wakanda speech," six weeks have passed, and the world is still in shock.
Learn more about Wakanda, refer to the link:
https://brainly.com/question/31162803
#SPJ1
A 0.682 g sample of a weak monoprotic acid, HA was dissolved in sufficient water to make 50.0 mL of solution and was titrated with a 0.135 molar NaOH solution. After the addition of 10.6 milliliters of base, a pH of 5.65 was recorded. The equivalence point was reached after the addition of 27.4 milliliters of the 0.135 molar NaOH.
a. Calculate the number of moles of acid in the original sample.
b. Calculate the molar mass of the acid HA.
c. Calculate the [H3O+] at pH = 5.65
d. Calculate the number of moles of unreacted HA remaining in solution when the pH was 5.65.
e. Calculate the value of the ionization constant, Ka, of the acid HA.
f. Calculate the value of the ionization constant, Kb, and explain how you would use it to determine the pH of a solution of a known mass of the sodium salt (Na)(A) dissolved in a known volume of water.
Answers
what is photosynthesis
Answers
Answer:
the process by which green plants and some other organisms use sunlight to synthesize foods from carbon dioxide and water. Photosynthesis in plants generally involves the green pigment chlorophyll and generates oxygen as a byproduct.
Explanation: You can put this in your own words. How? Well, try to find the most important stuff in your own words and write it down.
An aqueous potassium carbonate solution is made by dissolving 7.32 moles of K2CO3 in sufficient water so that the final volume of the solution is 4.80 L . Calculate the molarity of the K2CO3 solution.
Answers
According to the concept of molar concentration, molarity of the solution is 1.525 M.
What is molar concentration?Molar concentration is defined as a measure by which concentration of chemical substances present in a solution are determined. It is defined in particular reference to solute concentration in a solution . Most commonly used unit for molar concentration is moles/liter.
The molar concentration depends on change in volume of the solution which is mainly due to thermal expansion. Molar concentration is calculated by the formula, molar concentration=mass/ molar mass ×1/volume of solution in liters.
In terms of moles, it's formula is given as molar concentration= number of moles /volume of solution in liters.
In the given problem, by substituting values in mentioned formula related to moles we get,7.32/4.80=1.525 M.
Thus , the molarity of solution is 1.525 M.
Learn more about molar concentration,here:
https://brainly.com/question/21841645
#SPJ1
An atom with5 protons 6 neutrons and 5 electrons has an atomic mass of Amy
Answers
Answer: The number of neutrons for a given element is the only number that can change and still have the identity of the element stay the same, (because the atomic number is the number of protons). In this case the mass number would be 11.
Hope this helps...... Stay safe and have a Merry Christmas!!!!!! :D
I want to know how to solve this question by step by step.
Question: What is the pH value of 500ml of an aqueous solution of 0.005 mol HCl ?
Answers
The pH value of 500 mL of an aqueous solution of 0.005 mole HCl is 2
How do I determine the pH of the solution?We'll begin our calculations by obtaining the molarity of the solution. Details below:
Volume of solution = 500 mL = 500 / 1000 = 0.50 LMole of HCl = 0.005 moleMolarity = ?Molarity = mole / volume
Molarity = 0.005 / 0.50
Molarity = 0.01 M
Next, we shall obtain the concentration of the hydrogen ion, H⁺ in the solution. Details below:
HCl(aq) <=> H⁺(aq) + Cl¯(aq)
From the balanced equation above,
1 mole of HCl contains 1 mole of H⁺
Therefore,
0.01 M HCl will also contain 0.01 M H⁺
Thus, the concentration of the hydrogen ion, H⁺ in the solution is 0.01 M
Finally, we shall determine the pH of the solution. Details below:
Concentration of hydrogen ion [H⁺] = 0.01 MpH of solution =?pH = -Log H⁺
pH = -Log 0.01
pH = 2
Thus, we can conclude that the pH of the solution is 2
Learn more about pH:
https://brainly.com/question/22983829
#SPJ1
Someone help i need an answer quick

Answers
Claim 1: Yes, there probably will be a lunar eclipse of the moon of Kepler-47c during a year.
Claim 2: No, there probably won't be a lunar eclipse of the moon of Kepler-47c during a year.
What are the evidence to support this claim?Kepler-47c is a moon orbiting Kepler-47 AB, a binary star system. This implies that the moon's light varies on a regular basis as it circles the two stars. These changes in light might result in a lunar eclipse.
Lunar eclipses occur when the moon passes through the Earth's shadow. While Kepler-47c circles two stars, it is still subject to Earth's gravitational pull and may pass into its shadow.
Lunar eclipses occur on a regular basis, with two to four eclipses occurring on average per year. Given the frequency of lunar eclipses, Kepler-47c is likely to see at least one lunar eclipse every year.
Evidence to support this claim 2:
Lunar eclipses occur when the moon passes through the Earth's shadow. While Kepler-47c is in the Earth's gravitational field, it is not on the same orbital plane as the Earth.
While Kepler-47c circles two stars, its orbit is not known to be stable. Variations in Kepler-47c's orbit may reduce the likelihood of a lunar eclipse.
While lunar eclipses are common, they are not uniformly distributed throughout the year. The locations and motions of the Earth, moon, and sun govern the timing and frequency of lunar eclipses.
Find out more on Kepler-47c here: https://brainly.com/question/28741788
#SPJ1
match the number of negative charges to the number of positive charges to make each combination balanced (see image for answer) just say like thanks, or a fun fact or something for ten points in the answers lol

Answers
Answer : The correct match is:
1 positive charge = 1 negative charge
2 positive charges = 2 negative charges
3 positive charges = 3 negative charges
Explanation :
As we now that there are three subatomic particles which are protons, electrons and neutrons.
The protons and neutrons are located inside the nucleus and electrons are located around the nucleus.
The protons are positively charged, the electrons are negatively charged and neutrons are neutral.
As we know that all the things are made up of charges and opposite charges attract to each other.
In a neutral atom, the positive charges and negative charges are balanced in an object. That means, in neutral atom the number of positive charges are equal to the negative charges.
So we can say that:
1 positive charge = 1 negative charge
2 positive charges = 2 negative charges
3 positive charges = 3 negative charges
Answer:
1 positive---- 1 negative
2 positive---- 2 negative
3 positive---- 3 negative
Explanation:
edge 2023
PLEASE HELP ASAP
Use the equation below to answer the following questions.
2Al(s) + 3Cu(NO3)2(aq) 3Cu(s) + 2Al(NO3)3(aq)
Determine the oxidation state of the atoms in the equation's reactants and products: (6 points)
Oxidation state of Al in reactant:
in product:
Oxidation state of Cu in reactant:
in product:
Oxidation state of N in reactant:
in product:
Oxidation state of O in reactant:
in product:
Explain why this is a redox reaction.
Thank you!
Answers
Answer:
See explanation
Explanation:
A redox reaction equation shows a gain/loss of electrons from left to right in a reaction. In a redox reaction, a specie looses electrons while another specie gains electrons.
Considering the equation; 2Al(s) + 3Cu(NO3)2(aq) -----> 3Cu(s) + 2Al(NO3)3(aq)
The oxidation state of Al reactant is zero since the oxidation state of all uncombined elements is zero. The oxidation state of All in the product is +3
The oxidation state of the copper in the reactant is +2. The oxidation state of copper in the product is zero.
The oxidation state of N in the reactant and product is +5.
The oxidation state of oxygen in the reactant and product is (-2).
This is a redox reaction because from left to right, Al was oxidized (oxidation number increased from zero to +3) while Cu was reduced (oxidation number decreased from +2 to zero).
Consider the combustion of hexane:
2C6H14+19O2=12CO2+14H2O
a. If you started with 7.77 moles of hexane, how many of carbon dioxide could be produced?
b. If you started with 8.11g of hexane, how many moles of carbon dioxide could be produced?
c. How many grams of oxygen gas are needed to react with 25 moles of hexane?
d. What mass of water vapor, in kg, could be produced from combustion of 55.3kg of hexane?
Answers
a. As per the balanced reaction, 2 moles of hexane gives 12 moles of carbon dioxide. Then, 7.77 moles of hexane will give 46.2 moles of carbon dioxide.
What is combustion ?Combustion is an exothermic reaction in which a gas reacts with oxygen to give carbon dioxide and water. Given the combustion reaction of hexane gas.
b. molar mass of hexane = 86 g/mol
no.of moles in 8.11 g = 8.11 /86 = 0.094 g
2 moles of hexane gives 12 moles of carbon dioxide. Then 0.094 moles of hexane will give
0.094 × 12/2 = 0.56 moles
molar mass of carbon dioxide = 44 g/mol
then, mass of 0.56 moles = 0.56 × 44 = 24.6 g
c. 2 moles of hexane reacts with 19 moles of oxygen gas. Then, 25 moles of hexane needs to reacts with 237.5 moles of oxygen gas.
mass of oxygen gas = 32 g/mol
mass of 237.5 moles = 32 × 237.5 = 7600 g of oxygen is needed.
d. 2 moles of hexane gives 14 moles of water vapor. That is 172 g of hexane gives (18 × 14) 252 g of water.
then, 55.3 kg of hexane gives:
(55.3 kg × 0.252 kg)/0.172 kg = 81 kg.
Therefore, 81 kg of water vapor is produced from 55.3 kg of hexane.
Find more on combustion:
https://brainly.com/question/14987280
#SPJ1
Consider the following reaction:
2CH4(g)⇌C2H2(g)+3H2(g)
The reaction of CH4 is carried out at some temperature with an initial concentration of [CH4]=0.092M. At equilibrium, the concentration of H2 is 0.014 M.
Find the equilibrium constant at this temperature.
Answers
The equilibrium constant at this temperature is Kc= 4.17 x 10⁻⁶.
What is equilibrium?Since the equilibrium constant depends on the equilibrium concentration of both the reactants and the products of the chemical reaction.
Balanced reaction equation
2CH₄(g)⇌C₂H₂(g)+3H₂(g)
The initial concentration of the CH₄ = 0.093 M
The equilibrium concentration of the H = 0.017 M
Equilibrium constant = ?
Let's make the ice table
2CH₄(g) ⇌ C₂H₂(g) + 3H₂(g)
0.093 M 0 0
-2x +x +3x
0.093-2x x 0.017 M
3x = 0.017 M
Therefore, x =0.017 M /3 = 0.00567 M
Therefore, the equilibrium concentration of CH₄ =
0.093 M – 2x = 0.093 M – (2 x 0.00567 M) = 0.0817 M
Equilibrium concentration of the C₂H₄ = x = 0.00567 M
Let's write the equilibrium constant expression
Kc= [C₂H₄[H2]³/[CH₄]²
Let's put the values in the formula
Kc= [0.00567][0.017]³/[0.0817]²
Kc= 4.17 x 10⁻⁶
Therefore, the equilibrium constant is 4.17 x 10⁻⁶.
To learn more about equilibrium constant, refer to the link:
https://brainly.com/question/12971169
#SPJ9
How many grams of h2o are needed to produce 45g of NO
Answers
We need (i) the stoichiometric equation, and (ii) the equivalent mass of dihydrogen.
Explanation:
1
2
N
2
(
g
)
+
3
2
H
2
(
g
)
→
N
H
3
(
g
)
11.27
g
of ammonia represents
11.27
⋅
g
17.03
⋅
g
⋅
m
o
l
−
1
=
?
?
m
o
l
.
Whatever this molar quantity is, it is clear from the stoichiometry of the reaction that 3/2 equiv of dihydrogen gas were required. How much dinitrogen gas was required?
1)Grignard reagent when reacted with methanol will yield A) ethanol (B) secondary alcohols (C) tertiary alcohols (D ropanol (E) primary alcohol
Answers
When the reaction of Grignard reagent reacted with methanol will yield a tertiary alcohol. Therefore, Option C tertiary alcohol is correct.
Contains a carbon-metal link, Grignard reagents are chemicals used in catalysis. They generally result from the anhydrous reaction of magnesium metal with an alkyl or aryl halide. Because of their high reactivity, Grignard reagents frequently act as nucleophiles in organic reactions.
An alkyl group from a Grignard reagent binds to the oxygen atom of methanol (CH3OH) when it interacts with the methanol, breaking the carbon-metal connection. A precursor alkoxide is created as a result. The equivalent alcohol is then produced by protonating the intermediate alkoxide.
The reaction of a Grignard reagent with methanol leads to the formation of a tertiary alcohol.
Learn more about reagents here:
https://brainly.com/question/29729676
Suppose Gabor, a scuba diver, is at a depth of 15m15m. Assume that: The air pressure in his air tract is the same as the net water pressure at this depth. This prevents water from coming in through his nose. The temperature of the air is constant (body temperature). The air acts as an ideal gas. Salt water has an average density of around 1.03 g/cm3g/cm3, which translates to an increase in pressure of 1.00 atmatm for every 10.0 mm of depth below the surface. Therefore, for example, at 10.0 mm, the net pressure is 2.00 atmatm. What is the ratio of the molar concentration of gases in Gabor's lungs at the depth of 15 meters to that at the surface
Answers
Answer:
The ration of molar concentration is "2.5".
Explanation:
The given values are:
Average density of salt water,
= \(1.03 \ g/cm^3\)
Net pressure,
= \(2.00 \ atm\)
Increase in pressure,
= \(1.00 \ atm\)
Now,
The under water pressure will be:
= \(\frac{15 \ m}{10}\times 1 \ atm +1 \ atm\)
= \(1.5\times 1+1\)
= \(1.5+1\)
= \(2.5 \ atm\)
hence,
The ratio will be:
= \(\frac{(\frac{n}{V})_{15m} }{(\frac{n}{V})_{surface} }\)
or,
= \(\frac{P}{P_s}\)
= \(\frac{2.5}{1}\)
= \(2.5\)
write c or p to indicate weather each of the following is a chemical or physical property
Answers
Answer:
I think it's c
Explanation:
c seems like the best answer
Answer:
C since that has to be correct.
1.I make my cup of coffee in the morning by pouring boiling water over ground-up coffee beans held in a special piece of paper and by collecting the brown liquid that comes through. Which techniques of the five described in the background section is/are used in the preparation of the cup of coffee
Answers
Answer:
Filtration
Explanation:
Filtration is a physical separation technique that is used to separate solids from liquids or gases. The process involves the use of a filter which allows just the liquid to pass through.
In this case, the coffee beans is the solid and the boiling water is the liquid. The special piece of paper is the medium (filter) through which the coffee is collected.
The coffee coming through the special piece of paper (filter) is called a filtrate.
Filtration is used in the preparation of the cup of coffee.
If sulfur gained another electron, would its charge be positive or negative?
Explain your thinking. *
Answers
Answer:
AS WE KNOW THAT , when non-metallic elements gain electrons to form anions, SO sulphur is non metal and have the capacity to gain two electrons as lies in 6th group so it can gain electron and become sulphide ion(S-).
Thanks for asking questionExplanation:
Please help me ASAP!!!

Answers
240.1g is the mass of sodium propanoate. Mass was widely considered to be tied to the amount of matter within a physical body.
A body's mass is an inherent quality. Prior to the discoveries of the atom or particle physics, it was discovered that, despite having the same quantity of matter in theory, various atoms and elementary particles had varied masses. There are several conceptions of mass in contemporary physics that are theoretically different but practically equivalent. The resistance of the body to acceleration (alterations of velocity) in the presence of a net force may be measured experimentally as mass.
pH =Pka + log [salt]/[acid]
4.87=4.87+ log [salt]/[1]
4.87=4.87+ log [salt]/[1]
[salt] = 10 mol dm⁻³
mole = molarity ×volume
mole = 10×0.25
=2.5
mass = 2.5×96.07
= 240.1g
To know more about mass, here:
https://brainly.com/question/11954533
#SPJ1