Answers
Answer:
I'm pretty sure light waves do not require a medium to travel
Related Questions
the list which contains only metals is
Answers
Answer:
Hydrogen in its metallic state (usually considered a nonmetal)
Lithium.
Sodium.
Potassium.
Rubidium.
Cesium.
Francium.
Explanation:
Answer:
The list of elements that contains only metals is tin, copper and cesium.
Carbon and iodine aren't metals.
Helium isn't metal.
Neither iodine, carbon, nor argon are metals.
(Hope this helps) Sky
10. As the temperature of a fixed volume of a gas increases, the pressure will _______
answer is increase
Answers
f there is more than one possible site in the molecule/ion, focus on the central or the charged atom. a) b) c)
Answers
Answer:
This statement is in the context of Lewis Structures, which are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. The diagrams show the valence electrons of the atoms that participate in a bond.
When there is more than one possible site in the molecule or ion, the central or the charged atom must be focused on.
To determine which atom is the central or charged atom, you should look at the molecular/ionic structure and identify the atom with the highest electronegativity or charge density.
In general, the atom with the highest electronegativity or charge density is the central or charged atom.
Learn more about electronegativity here:
https://brainly.com/question/17762711#
#SPJ11
What volume of Co2 (carbon (iv) oxide)
will be produced when 10g of Na2Co3
(sodium trioxocarbonate (iv) reacted
with excess Hcl (Hydrogen Chloride) at
STP(Na=23, C=12, O=16)
Answers
Answer:
2.1056L or 2105.6mL
Explanation:
We'll begin by calculating the number of mole in 10g of Na2CO3. This can be obtained as follow:
Molar mass of Na2CO3 = (23x2) + 12 + (16x3) = 106g/mol
Mass of Na2CO3 = 10g
Mole of Na2CO3 =.?
Mole = mass /molar mass
Mole of Na2CO3 = 10/106
Mole of Na2CO3 = 0.094 mole
Next, we shall determine the number of mole CO2 produced by the reaction of 0.094 mole of Na2CO3. This is illustrated below:
Na2CO3 + 2HCl —> 2NaCl + H2O + CO2
From the balanced equation above,
1 mole of Na2CO3 reacted to produce 1 mole of CO2.
Therefore, 0.094 mole of Na2CO3 will also react to 0.094 mole of CO2.
Next, we shall determine the volume occupied by 0.094 mole of CO2 at STP. This is illustrated below:
1 mole of a gas occupy 22.4L at STP. This implies that 1 mole CO2 occupies 22.4L at STP.
Now, if 1 mole of CO2 occupy 22.4L at STP, then, 0.094 mole of CO2 will occupy = 0.094 x 22.4 = 2.1056L
Therefore, the volume of CO2 produced is 2.1056L or 2105.6mL
A scientist wants to know how individual lions within a pride interact with each other in their own environment.
To do this, the scientist sedates and tags all of the lions within a pride. Then, he places several remotely-controlled video cameras near the lions' den and performs an observational field study. He collects continuous video footage over the span of one year, analyzes the video, and then forms conclusions based on his observations.
This example shows that
A.
observational field studies are not a valid form of scientific investigation.
B.
conditions and variables are best controlled in observational field studies.
C.
there is only one way to acquire scientific knowledge.
D.
not all scientific knowledge is gained through controlled laboratory experiments.
Answers
Answer:
Answer Choice D
Explanation:
Study Island Question
Can anyone please answer questions 3 and 4
I’ll give the brainiest!
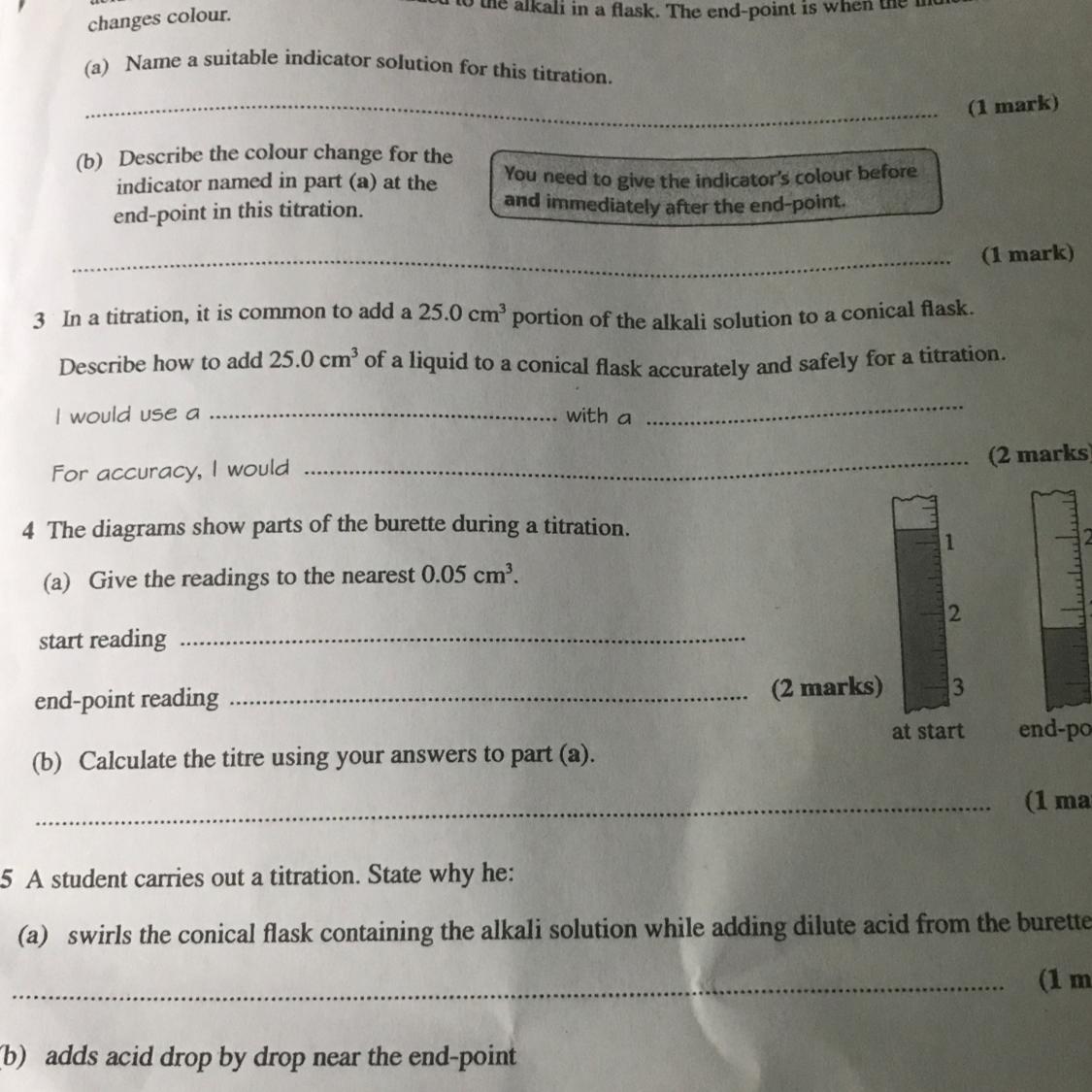
Answers
Answer:
1.Phenolphthalein
2.Method
a.Use the pipette and pipette filler to add 25 cm 3 of alkali to a clean conical flask.
b.Add a few drops of indicator and put the conical flask on a white tile.
c.Fill the burette with acid and note the starting volume.
d.Slowly add the acid from the burette to the alkali in the conical flask, swirling to mix.
Explanation:
Atomic masses of element
Hydrogen and carbon
Answers
Answer:
Atomic mass of Hydrogen is 1.00784 u
Atomic mass of Carbon is 12.0107 u
Explanation:
What is the independent variable of Smithers experience?
Please help controls and variables simpson? Smithers thinks that a special juice will increase the productivity of workers. He creates two groups of 50 workers each and assigns each group the same task (in this case, they're supposed to staple a set of papers). Group A is given the special juice to drink while they work. Group B is not given the special...
Answers
Answer:
Smithers thinks that a special juice will increase the productivity of workers. He creates two groups of 50 workers each and assigns each group the same task (in this case, they're ... Group A is given the special juice to drink while they work. Group ... 1. Control Group. Group B. 2. Independent Variable. volume of special juice.
Explanation:
Answer:
1. Group B
2. The juice
3. The number of stacks of paper
4. the juice did not help improve a quicker process
5. Group A can be given the juice, Group B can be given the juice without the special ingredient
USE QUIZLET FLASH CARDS IT CAN HELP YOU WITH A LOT OF QUESTION
Explain, in terms of the reaction rates, why the concentrations of the reactants and products remain constant in this system.
Answers
Explanation:
In a chemical reaction, the rates at which reactants are converted into products depend on various factors, including temperature, pressure, and concentration of the reactants. When the concentrations of reactants and products remain constant in a system, it typically indicates that the forward and reverse reaction rates are equal, resulting in a state of chemical equilibrium.
At equilibrium, the concentrations of reactants and products do not change over time, because the rates of the forward and reverse reactions are balanced. This occurs when the rate of the forward reaction, which converts reactants into products, is equal to the rate of the reverse reaction, which converts products back into reactants. As a result, the concentrations of both reactants and products remain constant.
The concept of Le Chatelier's principle can help explain why concentrations of reactants and products remain constant at equilibrium. According to Le Chatelier's principle, when a system at equilibrium is subjected to a change in temperature, pressure, or concentration, the system will adjust in a way that opposes the change. For example, if the concentration of a reactant is increased, the system will shift towards the side with fewer moles of reactant in order to restore the equilibrium. Similarly, if the concentration of a product is increased, the system will shift towards the side with fewer moles of product.
As a result of these shifts, the rates of the forward and reverse reactions will be adjusted to restore equilibrium, and the concentrations of reactants and products will remain constant. If the concentration of a reactant or product decreases, the system will shift in the opposite direction to restore equilibrium. This dynamic balancing of the forward and reverse reaction rates is what allows the concentrations of reactants and products to remain constant at equilibrium in a closed system.
An inflated balloon is left outside overnight. initially it has a volume of 1.84 L when the temperature is 293.4 K and the pressure. what temperature will the balloon have a volume of 1540mL. If the pressure falls to 14.41 psi?
Answers
Answer:
ffff
Explanation:
When 5,946 J of heat is added to 79.75 grams of oil at 37˚C , the temperature increases to 64˚C. What is the specific heat of the oil?
Answers
Answer:cool kid
Explanation:cool kid
La longitud de enlace del Bi—I experimental en el triyoduro de bismuto, BiI3, es 2.81 Å. De acuerdo con este valor y con los datos de la figura 7.7, prediga el radio atómico del Bi
Answers
Answer:
1,42 Å
Explanation:
La longitud de enlace de Bi-I se obtiene por
Bi-I = Radio del átomo de Bi + Radio del átomo de yodo
pero radio del átomo de yodo = 1.39Å
Longitud del enlace Bi-I = 2,81 Å
Por lo tanto, el radio del átomo de Bi = 2,81 Å - 1,39 Å
radio del átomo de Bi = 1,42 Å
What is a better conductor of electricity? Copper Iron Nickel Aluminum
Answers
I WILL GIVE YOU BRAINLIEST!!!

Answers
Answer:
The second option
Answer:
Intent to cause harm
The percentage of oxygen in NaOH is: a.16% b. 4% c.40% d. 8%
Answers
The percentage composition of oxygen in NaOH is 40%. The correct answer is C.
The formula for sodium hydroxide (NaOH) is Na + OH, which means it contains one atom of sodium (Na), one atom of oxygen (O), and one atom of hydrogen (H). The molecular weight of NaOH is 40 g/mol, which can be calculated by adding the atomic weights of its components: Na (23 g/mol) + O (16 g/mol) + H (1 g/mol).
The weight of oxygen in NaOH can be calculated by finding the weight of one oxygen atom and multiplying it by the number of oxygen atoms in one molecule of NaOH, which is one. The weight of one oxygen atom is approximately 16 g/mol.
Therefore, the weight of oxygen in NaOH is 16 g/mol. To calculate the percentage composition of oxygen in NaOH, we need to divide the weight of oxygen by the molecular weight of NaOH and multiply by 100.
(16 g/mol ÷ 40 g/mol) × 100% = 40%
Therefore, the answer is (c) 40%.
For such more questions on Oxygen
https://brainly.com/question/864285
#SPJ4
Note: The question would be as
What's the percentage composition of oxygen in NaOH is: a.16% b. 4% c.40% d. 8%
Solid iron(II) sulfide reacts with aqueous hydrochloric acid to produce aqueous iron(II) chloride and hydrogen sulfide gas . Write a balanced chemical equation for this reaction.
Answers
A balanced chemical equation for this reaction :
FeS(s)+2HCl(aq)→FeCl2(aq)+ H2S(g)
Further explanationGiven
Word equation
Required
Balanced equation
Solution
The chemical equation can be expressed in terms of:
word equation skeleton equation balanced equationSkeleton equation
FeS(s)+ HCl(aq) --> FeCl2 (aq) + H2S (g)
Balanced equation
FeS(s)+2HCl(aq)→FeCl2(aq)+ H2S(g)
How many half-lives are required for the concentration of reactant to decrease to 12. 5% of its original value?.
Answers
Answer:
Three half-lives
Explanation:
12.5% is one-eighth. One eighth is the cube of one half, so it would take three half-lives to reduce a reactant's concentration to 12.5%
Three half-lives are required for the concentration of reactant to decrease to 12. 5% of its original value.
What is half life?Half -life of a substance is defined as the time which is required for half of the quantity of a radioactive substance to get decayed.It is a term which is used in nuclear chemistry for describing how quickly unstable atoms undergo radioactive decay into other nuclear species by emitting particles or the time which is required for number of disintegrations per second of radioactive material to decrease by one half of its initial value.
In the given example 12.5 % is 1/8 and three times 1/2 that is 1/2×1/2×1/2=1/8 , hence three half lives are required for the concentration of reactant to decrease to 12. 5% of its original value.
Learn more about half-life,here:
https://brainly.com/question/24710827
#SPJ2
After Jenny finished collecting her data, she made the following table.
Toy Car Experiment
Distance Traveled
(meters) Total Time
(seconds)
10 7
20 14
30 21
40 28
50 35
60 42
Which of the following is true?
A.
It took the car less time to travel the first 10 meters than it did to travel the last 10 meters.
B.
The car was moving during the first 10 meters, but it was not moving during the last 10 meters.
C.
It took the car the same amount of time to travel the first 10 meters as it did to travel the last 10 meters.
D.
It took the car more time to travel the first 10 meters than it did to travel the last 10 meters.
Reset Submit
Answers
The true statement is: It took the car the same amount of time to travel the first 10 meters as it did to travel the last 10 meters (Option C)
How to determin which statement is trueThe following data were obtained from the question:
Distnce travelled: 10 20 30 40 50 60Time taken: 7 14 21 28 35 42Now, we shall determine the time taken to travel the first 10 m. This is shown below:
Distance = 10 - 0 = 10 mTime = 7 - 0 = 7 sNext, we shall determin the time taken to travel the last 10 m. This is shown below:
Distance = 60 - 50 = 10 mTime = 42 - 35 = 7 sFrom the above illlustration, we can see clearly that it took the car the same time to travel the first 10 m and the last 10 m.
Thus, the true statement is option C: It took the car the same amount of time to travel the first 10 meters as it did to travel the last 10 meters
Learn more about time:
https://brainly.com/question/17146782
https://brainly.com/question/17228876
#SPJ1
considering the dipole moment, choose the statement that is most accurate. choose one: a. the individual bonds are all nonpolar, so there are no individual dipoles in the molecules and, therefore, no net dipole moment. b. the o–cl bonds are all polar, so the molecules must have a net dipole moment. c. the o–cl bonds are all polar, but due to the linear shape of the molecules, the individual dipoles cancel to yield no net dipole moment for either molecule. d. the o–cl bonds are polar, but because the molecular structures are bent, the dipole moments do not cancel. the two molecules have identical dipole moments. e. the o–cl bonds are polar, but because the molecular structures are bent, the two molecules will have different dipole moments.
Answers
The most accurate statement considering the dipole moment is: c. The O-Cl bonds are all polar, but due to the linear shape of the molecules, the individual dipoles cancel to yield no net dipole moment for either molecule.
The most accurate statement considering the dipole moment is option c. In this case, the molecules in question have linear shapes, and all the O-Cl bonds are polar.
A polar bond occurs when there is an unequal distribution of electron density between two atoms, resulting in a separation of positive and negative charges. However, even though the O-Cl bonds are polar, the linear molecular structure leads to the cancellation of the individual dipole moments.
The dipole moment of a molecule is determined by both the magnitude and direction of its constituent bond dipoles. In this scenario, the linear shape causes the dipole moments to point in opposite directions, effectively canceling each other out.
As a result, there is no net dipole moment for either molecule. This cancellation of dipole moments due to molecular geometry is known as "vector sum" or "vector cancellation."
Thus, option c accurately describes the absence of a net dipole moment in the given molecules despite having polar O-Cl bonds.
Learn more about dipole moment from the given link:
https://brainly.com/question/11626115
#SPJ11
the mass of 2.000 L of glacial acetic acid, given that the density of this liquid is 1.049 g/cm3
Answers
Answer:17.47m
Explanation:
Explanation:
Molarity is defined as moles of solute per liters of solution.
c
=
n
V
Glacial acetic acid is actually anhydrous acetic acid, which implies that the acetic acid is not actually dissolved in a solvent and therefore is not ctually a solute.
However, you can still calculate its molarity based on the number of moles of you get per liter of glacial acetic acid.
To do that, start with a sample o
1.000 L
of glacial acetic acid. You know that at
25
∘
C
, glacial acetic acid has a density of
1.049 g/mL
, which tells you that every mililiter of glacial acetic acid has a mass of
1.049 g
.
This means that the mass of the sample will be
1.000
L
⋅
1000
mL
1.000
L
⋅
1.049 g
1
mL
=
1049 g
To find how many moles you get in the sample, use the given molar mass, which tells you how many grams of acetic acid you get per mole
1049
g
⋅
1 mole CH
3
COOH
60.05
g
=
17.469 moles CH
3
COOH
Now that you know the volume of the sample and how many moles it contains, you can say that
c
=
17.469 moles
1.000 L
=
17.47 M
I'll keep the number of sig figs given for the density of glacial acetic acid.
Which statement is true for a solution when its concentration of hydroxide ions becomes equal to the concentration of hydronium ions?
It becomes more acidic.
It becomes more alkaline.
Its pH becomes equal to 0.
Its pH becomes equal to 7.
Answers
When for a solution concentration of hydroxide ions becomes equal to the concentration of hydronium ions, its pH becomes equal to 7.
What is pH?pH of any solution is used to define the acidity, basicity or neutrality of the solution.
pH ranges from 0 to 6.9 shows acidity.pH 0 shows the neutrality.pH ranges from 7.1 to 14 shows the basicity.When the concentration of hydronium ion is equal to the concentration of the hydroxide ion then the resultant solution will be neutral, as they have a pH equals to 0.
Hence, pH of the solution becomes equal to 7.
To know more about pH, visit the below link:
https://brainly.com/question/172153
#SPJ1
What type of substance is reactant A?
Answers
Answer:
A reactant is a substance that is present at the start of a chemical reaction. The substance(s) to the right of the arrow are called products . A product is a substance that is present at the end of a chemical reaction.
Explanation:
What kind of bond will form when Sodium and Nitrogen react?
Answers
Answer: Ionic Bonds
"When it forms the nitride ion, it gains three electrons to form a 3- ion: N3− . In ionic compounds, the charges of constituent ions must balance. This can be achieved by having three sodium ions per nitride ion. Therefore, the formula of sodium nitride is Na3N"
Explanation:
https://socratic.org/questions/what-is-the-formula-for-sodium-nitride#:~:text=When%20it%20forms%20the%20nitride,nitride%20is%20Na3N%20.
for the formula c6h14 , calculate the index of hydrogen deficiency (ihd) and select all the types of unsaturation that might be present in the molecule based on the ihd.
Answers
The index of hydrogen deficiency (IHD) for the molecule C6H14 is 2, indicating the presence of two types of unsaturation, which can include double bonds, rings, or a combination of both.
The formula C6H14 represents a molecule with 6 carbon atoms and 14 hydrogen atoms. To calculate the index of hydrogen deficiency (IHD), we need to compare the actual number of hydrogen atoms in the molecule with the maximum number of hydrogen atoms possible for a molecule with the same number of carbon and heteroatoms.
The maximum number of hydrogen atoms for a saturated molecule can be calculated using the formula (2n + 2), where n is the number of carbon atoms. In this case, the maximum number of hydrogen atoms is (2 * 6 + 2) = 14.
The IHD can be calculated using the formula (2n + 2 - H)/2, where n is the number of carbon atoms and H is the actual number of hydrogen atoms in the molecule. Substituting the values, we get (2 * 6 + 2 - 14)/2 = 2.
The IHD of 2 suggests that there are two types of unsaturation point present in the molecule. The possible types of unsaturation include double bonds and rings. Therefore, the molecule with the formula C6H14 can have either double bonds or rings or a combination of both.
To know more about index of hydrogen deficiency (IHD) please refer:
https://brainly.com/question/29564213
#SPJ11
Assume that 0.950g of KHT (potassium hydrogen tartrate) are dissolved in 25.00mL of solution.KHT -> K++ HT-a) calculate the solubility of KHT for these conditions in g KHT / L of solutionb) Calculate the solubility of KHT for these conditions in mol KHT / L of solutionc) Determine [K+] and [HT-] in this solution. If the temperature is Tp, a trace of solid is present and the reaction is at equilibrium. Determine Ksp at this temperature
Answers
a) Solubility (g KHT/L) = (0.950 g KHT) / (0.025 L) = 38 g KHT/L
b) Solubility (mol KHT/L) = (38 g KHT/L) / (188.18 g/mol) = 0.202 mol KHT/L
c) [K+] = [HT-] = 0.202 mol KHT/L
d) Ksp = (0.202)(0.202) = 0.0408
A more detailed explanation of the answer.
a) To calculate the solubility of KHT in g KHT/L of solution, follow these steps:
1. Convert the volume of the solution to liters: 25.00 mL = 0.025 L
2. Calculate the solubility by dividing the mass of KHT by the volume of the solution:
Solubility (g KHT/L) = (0.950 g KHT) / (0.025 L) = 38 g KHT/L
b) To calculate the solubility of KHT in mol KHT/L of solution, follow these steps:
1. Determine the molar mass of KHT (K = 39.10 g/mol, H = 1.01 g/mol, C = 12.01 g/mol, O = 16.00 g/mol):
Molar mass of KHT = K + 2*(C + H + 2*O) = 39.10 + 2*(12.01 + 1.01 + 2*16.00) = 188.18 g/mol
2. Convert the solubility from g KHT/L to mol KHT/L:
Solubility (mol KHT/L) = (38 g KHT/L) / (188.18 g/mol) = 0.202 mol KHT/L
c) To determine [K+] and [HT-] in this solution, follow these steps:
1. Since KHT dissociates into K+ and HT-, the concentrations of K+ and HT- will be equal to the solubility of KHT in mol KHT/L:
[K+] = [HT-] = 0.202 mol KHT/L
As there is a trace of solid present and the reaction is at equilibrium, we can determine the Ksp at this temperature by following these steps:
1. Write the balanced chemical equation for the dissociation of KHT: KHT (s) <-> K+ (aq) + HT- (aq)
2. Write the expression for the Ksp: Ksp = [K+][HT-]
3. Plug in the concentrations calculated earlier: Ksp = (0.202)(0.202) = 0.0408
So, at this temperature (Tp), the Ksp for KHT is 0.0408.
Learn more about molar mass.
brainly.com/question/22997914
#SPJ11
if matter cant be created nor be destroyed so how was the universe was formed
Answers
Answer: By the very laws of the universe, matter cannot be created or destroyed, the Big Bang cannot have happened by its own power. There was a creator involved.
Complete and balance the following redox reaction in acidic solution H2O2(aq) + Cr2O72- (aq) → O2(g) + Cr3+ (aq)
Answers
The balanced redox reaction in acidic solution:
6H₂O₂(aq) + 2Cr₂O₇⁻²(aq) + 14H⁺(aq) → 3O₂ + 4Cr³⁺(aq) + 20H₂O(l)
To balance the redox reaction in acidic solution:
H₂O₂(aq) + Cr₂O₇⁻²(aq) → O₂(g) + Cr³⁺(aq)
We will follow the steps for balancing redox reactions in acidic solution:
Step 1: Assign oxidation numbers to all elements in the equation:
H₂O₂(aq): H has an oxidation state of +1, O has an oxidation state of -1.
Cr₂O₇⁻²(aq): Cr has an oxidation state of +6, O has an oxidation state of -2.
O₂(g): O has an oxidation state of 0.
Cr³⁺(aq): Cr has an oxidation state of +3.
Step 2: Identify the elements that are being oxidized and reduced:
Oxidation: Cr is being reduced from +6 to +3.
Reduction: H₂O₂ is being oxidized from -1 to 0.
Step 3: Write the half-reactions for oxidation and reduction:
Oxidation half-reaction: H₂O₂(aq) → O2(g)
Reduction half-reaction: Cr₂O₇⁻²(aq) → Cr³⁺(aq)
Step 4: Balance the atoms other than H and O in each half-reaction:
Oxidation half-reaction: 2H₂O₂(aq) → O₂(g)
Reduction half-reaction: Cr₂O₇⁻²(aq) → 2Cr³⁺(aq)
Step 5: Balance the oxygen atoms by adding water molecules (H2O) to the side that lacks oxygen:
Oxidation half-reaction: 2H₂O₂(aq) → O₂(g) + 2H₂O(l)
Reduction half-reaction: Cr₂O₇⁻²(aq) → 2Cr³⁺(aq)
Step 6: Balance the hydrogen atoms by adding hydrogen ions (H+) to the side that lacks hydrogen:
Oxidation half-reaction: 2H₂O₂(aq) → O₂(g) + 2H₂O(l)
Reduction half-reaction: Cr₂O₇⁻²(aq) + 14H+(aq) → 2Cr³⁺(aq) + 7H₂O(l)
Step 7: Balance the charges by adding electrons (e-) to the appropriate side of each half-reaction:
Oxidation half-reaction: 2H₂O₂(aq) → O₂(g) + 2H₂O(l) + 4e-
Reduction half-reaction: Cr₂O₇⁻²(aq) + 14H+(aq) + 6e- → 2Cr³⁺(aq) + 7H₂O(l)
Step 8: Multiply each half-reaction by a factor that will equalize the number of electrons in both half-reactions:
Multiply the oxidation half-reaction by 3 and the reduction half-reaction by 2:
6H₂O₂(aq) → 3O₂(g) + 6H₂O(l) + 12e-
Cr₂O₇⁻²(aq) + 14H+(aq) + 12e- → 4Cr³⁺(aq) + 14HvO(l)
Step 9: Combine the two half-reactions, canceling out the electrons on both sides:
6H₂O₂(aq) + 2Cr₂O₇⁻²(aq) + 14H⁺(aq) → 3O₂ + 4Cr³⁺(aq) + 20H
Step 10: Combine all the species to form the balanced redox reaction:
6H₂O₂(aq) + 2Cr₂O₇⁻²(aq) + 14H⁺(aq) → 3O₂ + 4Cr³⁺(aq) + 20H₂O(l)
Step 11: Simplify the equation by canceling out common species:
6H₂O₂(aq) + 2Cr₂O₇⁻²(aq) + 14H⁺(aq) → 3O₂ + 4Cr³⁺(aq) + 20H₂O(l)
Learn more about the redox reaction: https://brainly.com/question/28300253
#SPJ11
Suppose a solid object is fully immersed in a liquid, but B is larger than w. Is this possible at all
Answers
This problem is stating a situation in which a solid object is fully immersed in a liquid and the buoyant force is larger that its weight, so that you need to discuss whether this is possible or not.
In general terms, it is necessary to keep in mind that when the buoyant force is larger than its weight, the object will float. On the other side, when the buoyant force is smaller than the weight, then the object will sink.
It means that it could be possible to have this scenario under specific conditions. Now, the Archimedes' principle can be applied with the following version:
\(F_B+W>m_{obj}*a\\\\\rho _{fluid}*V*g+m_{obj}*g>m_{obj}*a\\\\\rho _{fluid}*V-m_{obj}>m_{obj}*a\)
It means that the object can move down the liquid if has a significant acceleration (could be external), even when the buoyant force is larger than the weight
Learn more:
https://brainly.com/question/18103369https://brainly.com/question/14271593write the net ionic equation for the acid-base hydrolysis equilibrium that is established when ammonium nitrate is dissolved in water.
Answers
The net ionic equation when the ammonium nitrate is dissolved in the water :
NH₄NO₃(s) + H₂O(l) ⇄ NH₄⁺(aq) + NO₃⁻(aq)
The component that will ionizes in the aqueous solution that is the ammonium ion. The nitrate ion is that does not ionize in the aqueous solution.
The acid-base hydrolysis in equilibrium that is the established when the ammonium nitrate is dissolved in the water, the net ionic equation is as :
NH₄NO₃(s) + H₂O(l) ⇄ NH₄⁺(aq) + NO₃⁻(aq)
The ions has the equal and the oppisite charges. They both can combine in the electrically neutral ratio of the 1:1. The net ionic equation can be depicts by the molecules and the ions.
To learn more about net ionic equation here
https://brainly.com/question/29299745
#SPJ4
5 isothermal expansion [5 pts] two moles of an ideal gas are expanded isothermally from an initial volume of 15 l to 20l l at a constant pressure of 1 atm. what is the work done on the gas in this case?
Answers
The work done on the gas in this case is 7.75 Joules or 7.75 L atm.
The work done by the gas in any thermodynamic process can be calculated by using the formula:
W = P∆V
In the isothermal process, the temperature of the gas remains constant, which means that the pressure and volume are inversely proportional to each other.
Therefore, the formula becomes:
W = nRTln(V2/V1)
W is the work done on the gasn is the number of moles of gas
R is the gas constant
T is the absolute temperature of the gas
V1 is the initial volume of the gas
V2 is the final volume of the gas
n = 2 moles
V1 = 15 L
V2 = 20
Lp = 1 atm
R = 0.0821 L atm/K mol
T = constant (since it's an isothermal process)
Therefore,W = (2 mol)(0.0821 L atm/K mol)(273 K) ln(20 L/15 L)
W = 7.75 L atm or 7.75 Joules
(since 1 L atm = 101.3 J)
Learn more about work -
brainly.com/question/33711659
#SPJ11
