Answers
Answer:
(a) Enantiomers
(b) Diastereomers
Explanation:
In the first pair of molecules, we have an opposite configuration. That is, in the first molecule we have an R and S configuration and in the second an S and R configuration. Therefore we have a mirror image, if this is true, we will have a mirror image and we will have "enantiomers". (See figure 1)
In the second pair of molecules, we do not have a mirror image. Since the first molecule has an R, S configuration (the mirror image would be S, R). In the second molecule, we have an R, R configuration (the mirror image would be S, S). Therefore, the relationship between these molecules is "diastereoisomers". (See figure 2)

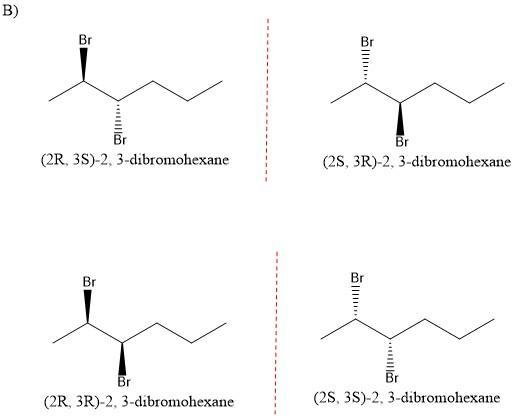
Related Questions
how the charge of an element is used to determine the chemical formula if the elements have equal and unequal charges.
Answers
Answer:
The charges are typically used to determine the mole ratio and composition of the individual atoms of elements in the compound
Explanation:
Take for instance the following compounds:
\(NaOH\) and \(H_{2} O\)
Na has a charge of +1 and OH -1. equal charge means equal composition.H has charge of +1 while O has charge of -2. The charges are unequal, so the mole ratio for conversion would require multiplication by 2...which makes H two atoms but oxygen one atom in the compound.I hope this helps.
At standard temperature and pressure conditions, the volume of an ideal gas contained in a jar is 55.3 L. How many molecules are in the jar. This question is to be answered in scientific notation.(eg. 1.5 e5)
Answers
Answer:
1.49e24
Explanation:
Standars temperature and pressure are 273.15K and 1atm, respectively.
Using ideal gas law, we can find moles of an ideal gas if we know its pressure, temperature and volume as follows:
PV = nRT
PV / RT = n
Where P is pressure (1atm), V is volume (55.3L), R is gas constant (0.082atmL/molK), T is temperature (273.15K) and n moles of the ideal gas.
Replacing:
PV / RT = n
1atm*55.3L / 0.082atmL/molK*273.15K = n
2.47 moles = n
Now, the question is about the number of molecules in the jar. By definition, 1 mole = 6.022x10²³ molecules.
As we have 2.47 moles:
2.47 mol × (6.022x10²³ molecules / 1 mole) =
1.49x10²⁴ molecules that are in the jar
In scientific notation:
1.49e24Move the chemistry book down again so it's surface touches the physics book. Quickly move the chemistry book back and forth. What's different when motion is faster?
Answers
Answer:
The temperature of the physics book increases as the chemistry book slides across it.
Explanation:
from plato
The temperature of the physics book increasing as the chemistry book slides across it is the difference when motion is faster.
What is Temperature?This is defined as the degree of hotness or coldness of a body and it increases when there is more friction.
When motion is faster , there is an increase in friction and increase in thermal(heat) energy which leads to increase in temperature.
Read more about Temperature here https://brainly.com/question/2331658
#SPJ2
The photon used to measure the electron location and
velocity has a size and velocity similar to that of the
electron and will displace the electron.
TRUE
FALSE
Answers
............................................................................................................................
Answers
Answer:
............................................................................................................................
Explanation:
because ........................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................
what is the most likely oxidation state of nitrogen
Answers
In comparing the chemistry of the amines with alcohols and ethers, we discover many classes of related compounds in which nitrogen assumes higher oxidation states, in contrast to limited oxidation states of oxygen. In this context, keep in mind that the oxidation state of elemental oxygen (O 2) and nitrogen (N 2) is defined as zero.
Answer:
-3
Explanation:
The reaction described by the equation O3(g)+NO(g)⟶O2(g)+NO2(g) has, at 310 K, the rate law
rate of reaction=[O3][NO]=3.0×106 M−1⋅s−1
Given that [O3]=4.0×10−4 M and [NO]=2.0×10−5 M at =0, calculate the rate of the reaction at =0
Answers
According to the question the rate of the reaction at =0 is 8.0 × 10−9 M2·s−1.
What is reaction?A reaction is an action or response to a stimulus. It can be a physical or mental response to the environment, or it can be a chemical reaction that occurs when two or more substances interact with each other. Reactions can be conscious or unconscious, and can involve emotions, memories, thoughts, or behaviors. All reactions are the result of some kind of change that occurs in the body or environment. Reactions can be beneficial or detrimental, depending on the situation. Learning how to recognize and control your reactions can help you make better decisions and lead a healthier, more successful life.
The rate of the reaction at =0 can be calculated by substituting the values of [O3] and [NO] into the rate law:
rate of reaction=[O3][NO]
= (4.0 × 10−4 M) (2.0 × 10−5 M)
= 8.0 × 10−9 M2·s−1
Therefore, the rate of the reaction at =0 is 8.0 × 10−9 M2·s−1.
To learn more about reaction
https://brainly.com/question/25769000
#SPJ1
Comment what type of fruit you like!
°ω°
Answers
What is pseudoscience? How is it different from non-science?
Answers
Pseudoscience is described as consisting of statements, beliefs, or practices that claim to be both scientific and factual but are incompatible with the scientific method.
The main difference between Pseudoscience and non- science is that pseudo-science is set up to look for evidence that supports its claims, while non-science do not look for evidence that might prove it false.
What is Pseudoscience?Pseudoscience is described as a proposition, a finding or a system of explanation that is presented as science but that lacks the rigor essential to the scientific knowledge.
A non-science on the other hand is an area of study that is not scientific, especially one that is not a natural science or a social science that is an object of scientific inquiry.
Learn more about Pseudoscience at: https://brainly.com/question/604092
#SPJ1
Write a persuasive essay stating which you believe is the most important Amendment to the U.S. Constitution: Amendment One, Four, or Six. Include three reasons to support your thesis.
Answers
Title: The First Amendment: Safeguarding Fundamental Freedoms
While all amendments are crucial, the First Amendment holds unparalleled significance in upholding the principles of liberty, equality, and democracy.
The First Amendment to the U.S. Constitution is undoubtedly the most important amendment as it safeguards fundamental freedoms that form the bedrock of a democratic society.
This amendment, which encompasses the rights of freedom of speech, religion, press, assembly, and petition, plays a vital role in protecting individual liberties and ensuring a just and inclusive society. There are three key reasons why the First Amendment stands out as the cornerstone of our democracy.
Firstly, freedom of speech allows citizens to express their ideas, opinions, and criticisms, fostering a robust marketplace of ideas essential for progress and social change. This right empowers individuals to challenge authority, hold public officials accountable, and engage in meaningful dialogue that drives societal progress.
Secondly, freedom of religion guarantees individuals the right to practice their faith without interference from the state. This principle promotes religious tolerance, diversity, and pluralism, creating a society where individuals can freely worship and live in accordance with their beliefs.
Lastly, freedom of the press ensures an informed citizenry by safeguarding independent journalism. A free press acts as a check on governmental power, exposes corruption, and provides essential information necessary for a functioning democracy.
For ore such questionns on Amendment visit:
https://brainly.com/question/28383565
#SPJ8
If 6.92 mol of C5H12 reacts with excess O2, how many moles of CO2 will be produced by the following combustion reaction? C5H12+8O2⟶6H2O+5CO2
Answers
If 6.92 mol of C₅H₁₂ reacts with excess O₂, number of moles of CO₂ will be produced by the following combustion reaction is 34.6 mol.
The balanced reaction is given as:
C₅H₁₂ + 8O₂ ---> 5CO₂ + 6 H₂O
Moles of C₅H₁₂ = 6.92 mol
1 mole of C₅H₁₂ produces 5 moles of CO₂
therefore , 6.92 mol of C₅H₁₂ = 5 × 6.92 mol
= 34.6 mol of CO₂
number of moles of CO₂ = 34.6 mol
Thus, If 6.92 mol of C₅H₁₂ reacts with excess O₂, number of moles of CO₂ will be produced by the following combustion reaction is 34.6 mol.
To learn more about moles here
https://brainly.com/question/15209553
#SPJ9
Write your answer using only positive exponents. ( 3m^4 n^4/6n^2)^3
Answers
Therefore, the final answer, with only positive exponents, is:\(27m^12 n^6.\)
First, we can simplify the expression inside the parentheses by canceling out the common factor :
Starting with the expression inside the parentheses:
\(3m^4 n^4/6n^2\)
We can simplify by dividing the numerator and denominator by 2:
\(3m^4 n^4/6n^2\)
= \((3/2) m^4 n^(4-2)\)
= \((3/2) m^4 n^2\)
Now we can raise this simplified expression to the power of 3 using the power of a product rule:
\([(3/2) m^4 n^2]^3 \\= (3/2)^3 m^(43) n^(23) \\= 27m^12 n^6\)
Therefore, the final answer is \(27m^12 n^6.\)
An exponent is a mathematical operation that represents repeated multiplication of a number by itself. It is written as a small number above and to the right of the base number. The exponent tells you how many times the base number is multiplied by itself. For example, in the expression 3^4, the base number is 3 and the exponent is 4, which means that 3 is multiplied by itself 4 times. The result of this operation is 81. Exponents are commonly used in algebra and calculus, as well as in many scientific and engineering fields to represent large and small numbers.
Learn more about exponent here:
https://brainly.com/question/5497425
#SPJ1
Write word equations for the following skeleton equations. 1. AI (s) + O2(g) AI2O3 (s)
Answers
Aluminum reacts with oxygen gas to produce aluminum oxide.
3. What does it mean if the Keq is <1?
Answers
Answer:
If the value of K is greater than 1, the products in the reaction are favored. If the value of K is less than 1, the reactants in the reaction are favored. If K is equal to 1, neither reactants nor products are favored.
what are thetypes of luminous flame
Answers
Types of luminous flames:
1. Yellow Luminous Flame
2. Smoky Luminous Flame
3. Orange Luminous Flame
4. Blue Luminous Flame
Luminous flames are characterized by their visible glow, which is caused by the incomplete combustion of fuel. The presence of soot particles in the flame causes the emission of light. There are different types of luminous flames, which can be classified based on their fuel composition and burning conditions. Here are some common types of luminous flames:
1. Yellow Luminous Flame: This is the most common type of luminous flame, often seen in open fires, candles, and gas stoves. It appears yellow due to the presence of soot particles in the flame. Yellow flames indicate incomplete combustion of hydrocarbon fuels, such as methane, propane, or natural gas. The high carbon content in these fuels leads to the formation of soot, which emits visible light.
2. Smoky Luminous Flame: This type of flame is characterized by a significant amount of black smoke and soot production. It is commonly observed in poorly adjusted or malfunctioning burners or engines. The excessive presence of unburned fuel in the flame results in incomplete combustion and the emission of dark smoke particles.
3. Orange Luminous Flame: An orange flame indicates a higher combustion temperature compared to a yellow flame. It is often seen in more efficient burners or when burning fuels with a higher carbon content, such as oil or diesel. The higher temperature helps in burning more of the carbon particles, reducing the amount of soot and making the flame appear less yellow.
4. Blue Luminous Flame: A blue flame is typically associated with complete combustion. It indicates efficient burning of fuel, resulting in minimal soot formation. Blue flames are commonly observed in gas burners or Bunsen burners. The blue color is a result of the combustion of gases, such as methane, in the presence of sufficient oxygen.
It's important to note that the luminosity of a flame can vary depending on factors such as fuel-air mixture, combustion temperature, and the presence of impurities. Achieving complete combustion and minimizing the production of soot is desirable for efficient and cleaner burning processes.
for more questions on luminous
https://brainly.com/question/27163038
#SPJ8
A mixture of fuel and is injected into a cylinder fitted with a piston. The initial volume is 0.37 L. After the mixture is ignited, gaseous products are formed and 1885 J of energy is released by the reaction. 311 J of the released energy is lost as heat to the surroundings. To what volume would the gases expand against a constant pressure of 1.036 atm, if the remainig energy is converted to work to push the
Answers
Answer:
The gases will expand to a volume of 2.37 L
Explanation:
Gases are able to do work when they expand or compress against an external constant pressure.
This work done by gases when they expand or compress against a constant external pressure is known as pressure-volume work or PV work.
The formula for calculating the work done by gases when they compress or expand against a constant pressure is given as W = PΔV
Where ΔV is change in volume given as V2 - V1
Where V2 is final volume, V1 is initial volume
By convention, W can either be negative or positive. When work is done by the system (ΔV > 0), W is negative and when work is done on the system (ΔV < 0), W is positive.
In the gas mixture above, W = PΔV
Remaining energy, W = (1885 - 311) J = 1574 J
P = 1.036 atm = 1.036 × 760 mmHg = 787.36 mmHg
ΔV = V2 - V1
ΔV = V2 - 0.37
1574 = 1.036 (V2 - 0.37)
V2 - 0.37 = 1574/787.36
V2 - 0.37 = 1.999 L
V2 = 1.999 + 0.37
V2 = 2.369 L
Therefore, the gases will expand to a volume of 2.37 L
What do you predict the chemical formula for the compound formed between calcium and sulfur?
Answers
Answer:
calcium donates two vanence electrons to sulfur atom to form Ca2+ ion and an S2+ - ion
1) To increase the amount of NH3 at 200 atm, the manufacturer should (increase, decrease, not change) the temperature of the reaction chamber.
2) This change in temperature would shift the reaction to the (left, right) because this equilibrium reaction is (exothermic, endothermic)
Answers
The temperature of the reaction should be decreased
This change in temperature would make the equilibrium to shift to the right.
What is the LeChatelier principle?
The Le Chatelier's principle, commonly referred to as the Le Chatelier's principle of equilibrium, is a chemical principle that describes how an equilibrium system reacts to environmental changes.
According to this theory, when an equilibrium system is exposed to an outside force, it will respond in a way that partially offsets the imposed change and restore equilibrium.
Learn more about LeChatelier principle:https://brainly.com/question/31377984
#SPJ1
scientist wants to use a model to help present the results of his detailed scientific investigation.
Why would a model be useful?
because the model makes the concepts easier to understand
because the model is easy to put together and to use
because the model prevents other scientists from asking questions
because the model requires the audience to pay full attention to it
Answers
Answer: A model would be useful because the model makes the concepts easier to understand.
Explanation:
Models are helpful tools in science education that can be used to enhance explanations, spark discussion, make predictions, provide visual representations of abstract concepts, and create mental models.
How is Carbon-12 and Carbon-13 different? What’s is the name that describes these two atoms?
Answers
Answer:
Carbon 12 and 13 are carbon isotopes, meaning that they have additional neutrons:
Carbon 12 has exactly 6 protons and 6 neutrons ( hence the 12 )
Carbon 13 has 6 protons and 7 neutrons ( hence the 13 )
They differ in the number of neutrons in the nucleus.
Names:
carbon-13, C-13
carbon-12, C-12
The specific gravity of a patient's urine sample was measured to be 1.008. Given that the density of water is 1.000 g/mL at 4°C, what is the density of the urine sample at 4°C
Answers
Answer:
1.008 g/mL
Explanation:
The specific gravity of any substance is the ratio of that substance's original density to the density of the referential substance, in this case, water at 4°C, which is 1.000 mL.
So we have the original density to be 1.008 g/mL, the specific gravity would also be 1.008 g/mL
The density of the urine sample at 4°C is 1.008 g/mL.
The specific gravity of a substance is the ratio of its density to the density of water. In this case, the specific gravity of the urine sample is 1.008, which means that its density is 1.008 times that of water.
Since the density of water is 1.000 g/mL, the density of the urine sample can be calculated as follows:
Density of urine sample = Specific gravity × Density of water
Density of urine sample = 1.008 × 1.000 g/mL
Density of urine sample = 1.008 g/mL
Learn more about specific gravity, here:
https://brainly.com/question/9100428
#SPJ1
Which of these waves has the greatest wavelength? (3 points) Wave shown with 2 wavelengths. Wave shown with 3 wavelengths. Wave shown with 1 wavelength stretch over a short distance. Wavelength shown with 1 wavelength stretched over a long distance.
Answers
The waves that has the greatest wavelength is Wavelength shown with 1 wavelength stretched over a long distance.
Waves explained.A wave could be a disturbance or variety that voyages through a medium or space, carrying vitality without transporting matter. Waves can take different shapes and happen totally different sorts of waves, counting mechanical waves and electromagnetic waves.
Mechanical waves require a medium to propagate, meaning they require a substance like water, discuss, or a strong fabric to transmit the wave. Illustrations of mechanical waves incorporate water waves, sound waves, and seismic waves. In these waves, particles of the medium sway or vibrate in a design, exchanging energy from one molecule to another.
Electromagnetic waves, on the other hand, don't require a medium and can travel through vacuum, such as in space. Electromagnetic waves comprise of electric and attractive areas swaying opposite to each other and to the heading of wave engendering. Illustrations of electromagnetic waves incorporate obvious light, radio waves, microwaves, infrared waves, bright waves, X-rays, and gamma beams.
Learn more about waves below.
https://brainly.com/question/26116832
#SPJ1
!!!!!PLEASE HELP!!!!!
On which of the following factors does the amount of energy absorbed by an endothermic reaction depend?
Number of reactants
Physical state of the reactant
Sum of the potential energy of the reactants and products
Difference in the potential energy of the reactants and products
Answers
Answer:
Difference in the potential energy of the reactants and products
Explanation:
The products have a lower potential energy than the reactants, and the sign of ΔH is negative. In an endothermic reaction, energy is absorbed. The products have a higher potential energy than the reactants, and the sign of ΔH is positive.
The factor on which the energy absorbed during an endothermic reaction depends upon is difference in the potential energy of the reactants and products.
What is an endothermic Reaction ?All the reactions that absorb energy from the environment, this energy is used as the activation energy required to carry out the reaction.
This reaction feels cold
In Endothermic Reaction the potential energy of the products that are being made is higher than the potential energy of the reactants.
The difference of the energy is taken from the environment or surrounding.
The difference is due to the bond strength of the reactants is more stronger than the bond strength in the products.
Strong bonds have lower potential energy than weak bonds.
Therefore the factor on which the energy absorbed during an endothermic reaction depends upon is difference in the potential energy of the reactants and products .
To know more about Endothermic Reaction
https://brainly.com/question/23184814
#SPJ2

what happens to water vapor when thermal energy is removed from it
Answers
Answer:
If thermal energy is removed from water vapor, it may start to change back into water, a liquid. This is because the thermal energy causes the gas particles to move around faster and more freely than solids or liquids, but if you take it away it may shift back to liquid/solid state.
Hope this helps :)
Explanation:
What does the Law of Conservation of Matter have to do with burning gasoline? Why is this crucial?
Answers
What mass of O₂ can be generated by the decomposition of 100.0 grams of NaCIO3? 2NACIO3 → 2NaCl + 30₂
A. 30.48 g
B.90.2 g
C.46.2 g
D. 45.1 g
Answers
Answer: D 45.1
Explanation:
100/106.441 x(3/2) x (31.9988) = 45.1
106.441 is the molar mass of NaClO3 from the periodic table
31.9988 is the molar mass of O2 from the periodic table
Which seasons in Atlanta GA have worst AQI
Answers
In Atlanta, GA, certain seasons are associated with poorer air quality due to various factors such as weather conditions, human activities, and geographical location.
Typically, the seasons with the worst AQI in Atlanta, GA, are summer and early fall. This is primarily due to the combination of high temperatures, stagnant air masses, and increased pollution from various sources.
During the summer months, Atlanta experiences hot and humid weather, which can contribute to the formation of ground-level ozone. Ozone is a harmful pollutant that is created when pollutants from vehicles, power plants, and industrial activities react with sunlight and heat. High levels of ozone can cause respiratory issues and other health problems.
In addition to ozone, Atlanta also experiences increased levels of particulate matter (PM) during the summer and early fall. PM refers to tiny particles suspended in the air, which can come from sources such as vehicle exhaust, industrial emissions, and wildfires.
These particles can be inhaled into the lungs and can have detrimental effects on respiratory health.
It's important to note that air quality can vary from year to year and is influenced by various factors. Local regulations, weather patterns, and changes in pollutant emissions can all impact the AQI during different seasons.
Monitoring air quality reports and taking necessary precautions such as reducing outdoor activities during times of poor air quality can help individuals stay informed and protect their health.
For more such question on air quality visit:
https://brainly.com/question/21173066
#SPJ8
Analyze This: The ionic bond between sodium (Na) and sulfur (S) forms as a
result of e transfer. One element donates or gives away one or more e's. The
other element accepts or receives the e's.
To begin, identify the e shell
diagram for sodium and for
sulfur:
Next
Na:
S:
Answers
To form ionic bonds, atoms transfer electrons. Consider sodium as: It is permanent in its anionic form and has one valence electron.
Ionic linkage Why does that matter?Ionic connections are created when specific electrons are fully transferred from one atom to another. When atoms lose one or more electrons, cations are produced (positively charged ions). When an atom receives one or more electrons, it produces an anion (negatively charged ion) .
Ionic bonding is demonstrated via the synthesis of sodium fluoride (NaF) from sodium and fluorine atoms. The sodium atom's lone valence electron is accepted in this process by a fluorine atom that has just enough area to accommodate it. Ions or charged molecules are referred to as "ionic" compounds. Ionic bonds are the interaction between ions with different charges. If we glance at the adjective, ionic, we can tell that the subject is science. Ionic bonds hold two or more atoms together to form ionic molecules.
For more information on bonding, see:
brainly.com/question/819068
#SPJ1
Which line represents the motion of the cathode ray in an electric field?

Answers
The line representing the motion of the cathode ray in an electric field is line A.
option C.
What is cathode ray
Cathode rays are streams of electrons that are emitted from the negatively charged electrode, or cathode, in a vacuum tube. They were first discovered by British physicist J.J. Thomson in the late 19th century.
Cathode rays are produced when a voltage is applied across two electrodes in a vacuum tube, and the cathode is heated. The electrons are then accelerated towards the anode, or positively charged electrode, and collide with gas molecules in the tube. These collisions produce a visible stream of electrons that can be seen using fluorescent materials.
Cathode rays have played an important role in the development of electronics, particularly in the early years of television and computer displays. The first television sets used cathode ray tubes (CRTs) to display images, and cathode ray oscilloscopes are still used today in electronics and engineering applications.
Learn more about cathode ray here: https://brainly.com/question/4441361
#SPJ1
what volume of 7.25 mol/L stock solution in needed to make 3.84 L of 8.50 mol/L solution?
Answers
Answer:
4.50 L
Explanation:
First we calculate how many moles are there in 3.84 L of a 8.50 mol/L solution:
3.84 L * 8.50 mol/L = 32.64 molNow, keeping in mind that
Concentration = Mol / Volumewe can calculate the volume of a 7.25 mol/L solution that would contain 32.64 moles:
Volume = Mol / ConcentrationVolume = 32.64 mol ÷ 7.25 mol/LVolume = 4.50 LSo we could take 4.50 L of the 7.25 mol/L solution and evaporate the solvent until only 3.84 L remain.