Explain how a trait is passed down from one generation to the next
Answers
Answer:
one generation to the next via DNA,
Explanation:
Heritable traits are known to be passed from one generation to the next via DNA, a molecule that encodes genetic information. ... Organisms inherit genetic material from their parents in the form of homologous chromosomes, containing a unique combination of DNA sequences that code for genes.
Related Questions
what is electrical energy
Answers
Electrical energy is a energy resulting from electric charges or existing kinetic energy. It does work or applies force to move an object, using electricity. In the case of electrical energy, the force is electrical attraction or repulsion between charged particles.
Which of the following contains factors that all affect polymer properties? A. chain length, intermolecular forces, and the molar mass of the monomers B. chain length, the C-H bond strength, and the extent of chain branchingC. chain length, intermolecular forces, and the strength of C- H bonds D. chain length, intermolecular forces, and the extent of chain branching
Answers
The answer is D. Chain length, intermolecular forces, and the extent of chain branching all affect polymer properties.
Chain length influences a polymer's mechanical and physical properties, such as tensile strength and toughness. As the chain length increases, the polymer becomes more resistant to deformation. Intermolecular forces, including van der Waals forces, hydrogen bonding, and dipole-dipole interactions, play a crucial role in determining a polymer's thermal, mechanical, and solubility properties. Polymers with strong intermolecular forces exhibit higher melting points and increased mechanical strength.
Finally, the extent of chain branching impacts a polymer's properties by influencing its crystallinity, density, and molecular weight distribution. Highly branched polymers have lower crystallinity and density, which can result in reduced mechanical strength and increased permeability. Understanding these factors is essential for designing polymers with specific desired properties for various applications.
Learn more about polymers here:
https://brainly.com/question/1443134
#SPJ11
The solubility product constant at 25°C for AgI(s) in water has the value 8.3 × 10–17. Calculate ∆Grxn at 25°C for the process AgI(s) <--> Ag+(aq) + I– (aq) where [Ag+] = 9.1 × 10–9 and [I–] = 9.1 × 10–9. –91.7 kJ/mol +91.7 kJ/mol 0.0 kJ/mol –4.4 kJ/mol +4.4 kJ/mol
Answers
Answer:
+91.7 KJmol-1
Explanation:
Recall that ∆G= -RTlnK
Since ∆G in this case is ∆Grxn and K is the Ksp
Note that the Ksp is the solubility product (as shown by the reaction equation)
∆Grxn is the change in free energy for the reaction, in this case the ionization of the silver iodide into silver and iodide ions.
R= 8.314JK-1 and T =25°C +273 = 298 K (the centigrade temperature must be appropriately converted to its corresponding absolute absolute before proceeding with the calculation)
Hence we can substitute values accordingly;
∆Grxn = -(8.314 × 298 × ln 8.3×10^-17)
∆Grxn = +91.7 KJmol-1
Consider a 0.12 M solution of a weak polyprotic acid (H2A) with the possible values of Ka1 and Ka2 given here. Calculate the contributions to [H3O+] from each ionization step. At what point can the contribution of the second step be neglected?
A. Ka1=1.0×10−4 and Ka2=5.0×10−5
Answers
The [H3O+] in step 1 is 0.0034 M while the [H3O+] in step 2 is 0.00039 M
What is the contribution of each step?Let us set up the ICE table in each case, for K1;
H2A(aq) + H2O(l)--------> H3O^+(aq) + HA^-(aq)
I 0.12 0 0
C -x +x +x
E 0.12 - x x x
Ka1= [H3O^+] [HA^-]/[ H2A]
Ka1= x^2/ 0.12 - x
1.0×10^−4 = x^2/ 0.12 - x
1.0×10^−4(0.12 - x ) = x^2
1.2 * 10^-5 - 1.0×10^−4x = x^2
x^2 + 1.0×10^−4x - 1.2 * 10^-5 = 0
x =0.0034 M
[H3O+] = 0.0034 M
Again; [H3O+] = [HA^-] = 0.0034 M
HA^-(aq) + H20(l) -------> A^-(aq) + H3O^+
I 0.0034 0 0
C -x + x +x
E 0.0034 - x x x
Ka2= [A^-] [H3O^+]/[HA^-]
5.0×10^−5 = x^2/ 0.0034 - x
5.0×10^−5 (0.0034 - x ) = x^2
1.7 * 10^-7 - 5.0×10^−5x = x^2
x^2 + 5.0×10^−5x - 1.7 * 10^-7 = 0
x=0.00039 M
Learn more about the dissociation of a polyprotic acid:https://brainly.com/question/14481763
#SPJ1
State two substances that can be used as a filter in water treatment.
Answers
Answer:
1. pure chlorine
2.chloramine
Explanation:
Water's unusual properties are primarily due to
a molecular geometry
b small molar mass
c chemical reactivity
d hydrogen bonding
Answers
Answer:
D hydrogen bonding
Explanation:
solve for x 5 to the power 2 x + 4 - 25 to the power x - 1 is equals to
Answers
Answer:
[][][][][][][][][][][][][][]
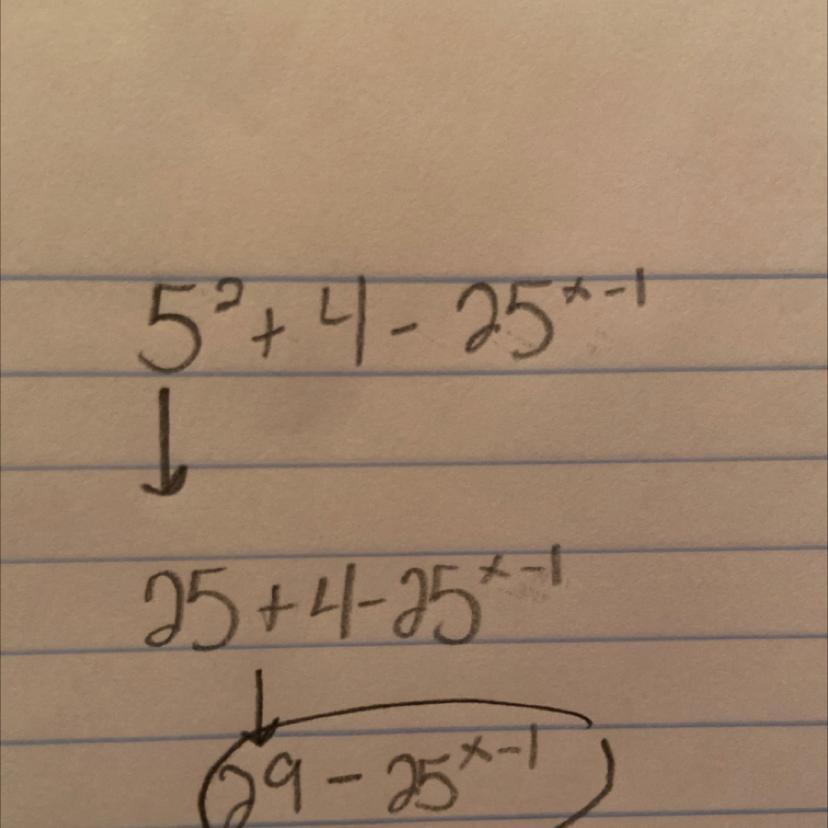
ethylene gas is an organic molecule that serves
Answers
As a plant hormone, specifically as a signaling molecule that regulates various growth and development processes. It is involved in the process of fruit ripening, senescence, and the transition from the vegetative to the reproductive phase of the plant life cycle.
Ethylene gas is produced by plants in response to various stimuli, such as wounding, senescence, or the approach of the end of the day. It acts as a messenger molecule that triggers a cascade of biochemical reactions in the plant cells, leading to the breakdown of cell walls, softening of tissues, and the onset of ripening.
Ethylene gas is a potent hormone that affects various aspects of plant growth and development, such as fruit ripening, senescence, dormancy, and flowering. It plays a critical role in plant responses to environmental signals and stresses, such as drought, pathogens, and temperature changes.
Overall, ethylene gas is an important molecule that helps plants adapt to changing environmental conditions and ensures their survival and reproduction.
Learn more about molecule visit: brainly.com/question/1078183
#SPJ11
A farmer wants to build a pond for her cows. What step must she take in order to build the pond?
A. Dig into the zone of aeration.
B. Place a permeable material on the ground.
C. Keep the soil moist.
D. Place an impermeable material on the ground.
Answers
Answer:
Option A:
Dig into the zone of aeration
Explanation:
Within the lithosphere of the earth's surface, the zone of aeration is the zone directly above the water table, with a lot of pore spaces within the rocks. These pore spaces contain air and water, which can be used to water the cows.
Once the zone of aeration has been dug into, the farmer can tap into the underground reserve of water, which is stored within the pores of rocks. This water can now sip into the hole that was dug up by the farmer, forming a pond for the cows.
PPPPPPPPPPPPPPPLLLLLLLLLLLLLLLLLEEEEEEEEEEEEEEEEEESSSSSSSSSSSSEEEEEE
HHHHHHHHHHHHHHHHHHHHHHHEEEEEEEEEEEEEEEEELLLPPPPPPPPPPPPPPPPPPMMMMMMMMMMEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEE!
Answers
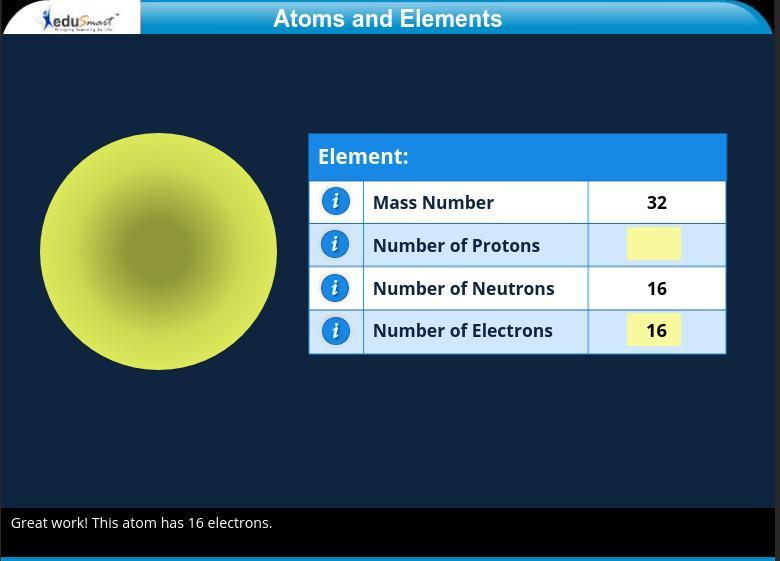
Explanation: you subtract the neutrons from the mass number so 32-16=16
Answer:
Explanation:
The answer is 16
Which best describes the trends in electronegativity on the periodic table?
Electronegativity increases up and to the right.
Electronegativity increases down and to the left.
Electronegativity decreases up and to the left.
Electronegativity decreases down and to the right.
Answers
Answer:
A. Electronegativity increases up and to the right.
Explanation:
edge 2021
Electronegativity increases up and to the right. Hence, option A is correct.
What is a periodic table?The periodic table is a tabular arrangement of the chemical elements organized with an increasing atomic number.
Electronegativity is a chemical property that explains the trend of an atom to attract a shared pair of electrons towards itself. As you move from left to right across the periodic table, electronegativity increases, and as you move down the table electronegativity decreases.
Hence, option A is correct.
Learn more about the periodic table here:
brainly.com/question/11155928
#SPJ5
How many moles is 1.8 x 1023 atoms of Be?
Answers
Answer:
3.0059572661984113e-24
Explanation:
2nd time posting someone please help

Answers
Answer:
Sb: Antimony
Si: 14 (atomic Number)
S: 32.06 u (atomic mass)
Explanation:
NO LINKS please help!

Answers
Answer:
True True False
Explanation:
Malonate is a competitive inhibitor of succinate dehydrogenase. If malonate is added to a mitochondrial preparation that is oxidizing pyruvate as a substrate, which compound would you expect to decrease in concentration?.
Answers
Fumarate is expected to decrease in concentration, If malonate is added to a mitochondrial preparation that is oxidizing pyruvate as a substrate.
Only succinate dehydrogenase is a membrane-bound TCA cycle enzyme; all other enzymes are dispersed throughout the matrix of the mitochondria. This enzyme participates in both the electron transport chain and the TCA. With the conversion of FAD to FADH2, the succinate dehydrogenase enzyme in TCA transforms succinate into fumarate. The reaction's cofactor and electron acceptor is FAD.
Succinate dehydrogenase is an enzyme complex anchored to the inner mitochondrial membrane that facilitates the Krebs cycle's oxidation of succinate to fumarate and the electron transport chain's concomitant reduction of ubiquinone to ubiquinol.
To know more about Fumarate visit : https://brainly.com/question/13316520
#SPJ4
enter the compound that forms between lithium and carbonate.
Answers
The compound that forms between lithium and carbonate is lithium carbonate (Li₂CO₃).
Lithium carbonate (Li₂CO₃) is a chemical compound that forms when lithium (Li) reacts with carbonate (CO₃). It is an important lithium compound with various applications and properties.
Lithium, a highly reactive alkali metal, readily reacts with carbonate ions to form lithium carbonate. The reaction can be represented by the following equation:
2Li + CO₃ → Li₂CO₃
Lithium carbonate is a white crystalline solid that is sparingly soluble in water. It has a molecular weight of 73.89 g/mol and a density of 2.11 g/cm3. The compound has a high melting point of approximately 723°C (1,333°F), making it useful in high-temperature applications.
One of the primary applications of lithium carbonate is in the production of lithium-ion batteries, which are widely used in electronic devices, electric vehicles, and renewable energy storage systems. Lithium carbonate is a key raw material in the synthesis of lithium cobalt oxide (LiCoO₂), a cathode material used in lithium-ion batteries. It helps enhance the battery's energy density and performance.
Lithium carbonate also has applications in the pharmaceutical industry. It is used as a mood stabilizer and for the treatment of bipolar disorder and depression. The compound helps regulate the levels of certain neurotransmitters in the brain, contributing to its therapeutic effects.
Learn more about Alkali Metals at
brainly.com/question/18534253
#SPJ4
Calculate the pH and the equilibrium concentration of S²- in a 6.89x10-2 M hydrosulfuric acid solution, H₂S (aq). For H₂S, Ka1 = 1.0x10-7 and Ka_2 = 1.0×10-1⁹ pH = [S²] = M
Answers
Therefore, the pH and the equilibrium concentration of S²⁻ in a 6.89x10⁻² M hydrosulfuric acid solution are pH = 7.78 and [S²⁻] = 2.31x10⁻¹¹ M.
Hydrosulfuric acid (H₂S) is a weak acid that dissociates in water to produce hydrogen ions (H⁺) and bisulfide ions (HS⁻). H₂S(aq) + H₂O(l) ⇌ H₃O⁺(aq) + HS⁻(aq)
The bisulfide ions (HS⁻) in turn reacts with water to produce hydronium ions (H₃O⁺) and sulfide ions (S²⁻).
HS⁻(aq) + H₂O(l) ⇌ H₃O⁺(aq) + S²⁻(aq) Ka1
= 1.0x10⁻⁷,
Ka2 = 1.0x10⁻¹⁹
To calculate the pH and the equilibrium concentration of S²⁻ in a 6.89x10⁻² M H₂S(aq) solution, we must first determine if H₂S(aq) is a strong or weak acid.
It has Ka1 = 1.0x10⁻⁷, which is a very small value; thus, we can conclude that H₂S(aq) is a weak acid.
To calculate the equilibrium concentration of S²⁻ in a 6.89x10⁻² M H₂S(aq) solution, we need to use the Ka2 value (Ka2 = 1.0x10⁻¹⁹) and a chemical equilibrium table.
[H₂S] = 6.89x10⁻² M[H₃O⁺] [HS⁻] [S²⁻]
Initial 0 0 0Change -x +x +x
Equilibrium (6.89x10⁻² - x) x xKa2 = [H₃O⁺][S²⁻]/[HS⁻]1.0x10⁻¹⁹
= x² / (6.89x10⁻² - x)
Simplifying: 1.0x10⁻¹⁹ = x² / (6.89x10⁻²)
Thus: x = √[(1.0x10⁻¹⁹)(6.89x10⁻²)]
x = 2.31x10⁻¹¹ M
Thus, [S²⁻] = 2.31x10⁻¹¹ M
To calculate the pH of the solution, we can use the Ka1 value and the following chemical equilibrium table.
[H₂S] = 6.89x10⁻² M[H₃O⁺] [HS⁻] [S²⁻]
Initial 0 0 0
Change -x +x +x
Equilibrium (6.89x10⁻² - x) x x
Ka1 = [H₃O⁺][HS⁻]/[H₂S]1.0x10⁻⁷
= x(6.89x10⁻² - x) / (6.89x10⁻²)
Simplifying: 1.0x10⁻⁷ = x(6.89x10⁻² - x) / (6.89x10⁻²)
Thus: x = 1.66x10⁻⁸ M[H₃O⁺]
= 1.66x10⁻⁸ M
Then, pH = -log[H₃O⁺]
= -log(1.66x10⁻⁸)
= 7.78 (rounded to two decimal places)
To know more about concentration visit:
https://brainly.com/question/30862855
#SPJ11
How many molecules of carbon dioxide would be produced by five turns of the citric acid cycle?.
Answers
10 molecules of the Carbon-dioxide (CO₂) would be produced by five turns of citric acid cycle (TCA).
A group of the two or more atoms forms the smallest identifiable unit into which the pure substance can be divided and it still retain the composition and chemical properties of that substance.
The citric acid cycle is a continuous series of reactions that produces 2 carbon-dioxide molecules, one GTP/ATP molecule, and the some reduced forms of NADH, in one turn of the citric acid cycle.
Taking into account only the citric cycle, the two molecules are created in a single turn when the acetyl coA enters the citric acid cycle.
The citric acid cycle will generate ten molecules of carbon dioxide in five turns.
Thus, 10 molecules of Carbon-dioxide would be produced by five turns of citric acid cycle (TCA).
To learn more about Citric Acid Cycle, please refer:
brainly.com/question/17089080
#SPJ4
What mass of LiOH would need to be dissolved in water to make 300.0 mL of a solution with a pH of 11.33?
Answers
Execute Order 66________________
The mass of LiOH needed to make a 300 mL of a solution with a pH of 11.33 is 0.015 g
We'll begin by calculating the pOH of the solution.
pH = 11.33
pOH =?
pH + pOH = 14
11.33 + pOH = 14
Collect like terms
pOH = 14 – 11.33
pOH = 2.67 Next, we shall determine the concentration of hydroxide ion, OH⁻pOH = 2.67
Concentration of hydroxide ion [OH⁻] = ?pOH = –Log [OH⁻]
2.67 = –Log [OH⁻]
–2.67 = Log [OH⁻]
Take the as antilog of –2.67
[OH⁻] = antilog (–2.67)
[OH⁻] = 0.0021 MNext, we shall determine the concentration of LiOH.LiOH(aq) —> Li⁺(aq) + OH⁻(aq)
From the balanced equation above,
1 mole of LiOH contains 1 mole of OH⁻
Therefore,
0.0021 M LiOH will also contain 0.0021 M OH⁻.
Next, we shall determine the mole of LiOH in the solution.Molarity of LiOH = 0.0021 M
Volume = 300 mL = 300 / 1000 = 0.3 L
Mole of LiOH =?Mole = Molarity × volume
Mole of LiOH = 0.0021 × 0.3
Mole of LiOH = 0.00063 moleFinally, we shall determine the mass of LiOH needed to prepare the solution.Mole of LiOH = 0.00063 mole
Molar mass of LiOH = 7 + 16 + 1 = 24 g/mol
Mass of LiOH = ?Mass = mole × molar mass
Mass of LiOH = 0.00063 × 24
Mass of LiOH = 0.015 gTherefore, the mass of LiOH needed to prepare the solution is 0.015 g
Learn more: https://brainly.com/question/5851093
If Half- life of an isotope is 30 days and it was assumed that
the person ate 100 Bq of isotope. Using the GI track model
information, calculate the number of transformations in
Stomach
Answers
If Half- life of an isotope is 30 days and it was assumed that the person ate 100 Bq of isotope, there are 50 transformations in the stomach.
The radioactive decay of a sample of an isotope can be characterized by the half-life of that isotope. When a radioisotope undergoes decay, its nucleus becomes unstable, and it emits particles or energy to become more stable. The half-life of an isotope is the time it takes for half of the original sample to decay. The question states that the half-life of an isotope is 30 days, and the person ingested 100 Bq of isotope. It also says to calculate the number of transformations in the stomach using GI track model information .
Since the isotope has a half-life of 30 days, we can use the following formula to find the number of transformations in the stomach:` N = N₀ (1/2)^(t/T₁/₂)`where: N₀ = initial number of nuclei N = final number of nuclei (after time t)T₁/₂ = half-life of the isotope The isotope has a half-life of 30 days, so T₁/₂ = 30 days. The question doesn't specify how long the person has had the isotope in their stomach, so we'll assume it's been there for one half-life, or 30 days. Therefore, t = 30 days.
Substituting into the formula:` N = 100 (1/2)^(30/30)`Simplifying:` N = 100 (1/2)^1`Evaluating:`N = 50`So after 30 days in the stomach, the person would have 50 Bq of the isotope left. Therefore, the number of transformations in the stomach is the difference between the initial number of transformations (100 Bq) and the final number of transformations (50 Bq):`Number of transformations in stomach = 100 - 50 = 50 transformations. Therefore, there are 50 transformations in the stomach.
More on isotope: https://brainly.com/question/28039996
#SPJ11
a chemist has synthesized a greenish-yellow gaseous compound that contains only chlorine and oxygen and has a density of 7.71 g/l at 36.0 degrees celsius and 2188.8 mm hg. what is the molar mass of the compound?
Answers
b.c10²
i dont know if it is right sorry if isn't
the best reactants to convert cyclohexanone to 2-methylcyclohexanone cleanly (with minimal side reactions) would be:
Answers
By use of a Friedel-Crafts alkylation reaction, cyclohexanone can be changed into 2-methylcyclohexanone.
An arene (in this example, cyclohexanone) reacts with an alkyl halide (in this case, a methyl halide) to produce the desired product (in this case, 2-methylcyclohexanone) in the presence of a Lewis acid catalyst, such as aluminium chloride.However, employing this reaction to change cyclohexanone into 2-methylcyclohexanone has certain drawbacks. Controlling the reaction's regioselectivity, or making sure the methyl group is transferred to the proper location on the cyclohexanone ring to create 2-methylcyclohexanone, is one of the key hurdles. Minimising the development of undesirable side products, such as the di- or self-alkylated product, is another difficulty.To clarifyThe primary alkyl halide methyl iodide (CH3I) is an excellent candidate for the alkylating agent since it reacts more quickly and with higher selectivity than secondary or tertiary alkyl halides. Additionally, it is a fantastic departing group that encourages an effective response.Using aluminium chloride (AlCl3) as a catalyst for a Lewis acid It is well known that aluminium chloride is a potent catalyst for the alkylation of arenes in Friedel-Crafts processes. Additionally, it is widely accessible and reasonably priced.With these reactants, it is possible to optimise the reaction conditions to favour the desired alkylation reaction while minimising the production of undesirable side products. For instance, to favour the mono-alkylation product over other products, the reaction can be carried out at low temperature (-78°C).
learn more about methylcyclohexanone here
https://brainly.com/question/31994079
#SPJ11
Which term is used to describe the variety of inheritable traits in a species? (4 points)
Ecosystem diversity
Genetic diversity
Natural selection
Species diversity
Answers
Answer:
B Genetic diversity
Explanation:
Answer:
I think the answer is b.
Explanation:
:)
What is the volume of 6.9 mol of oxygen at 233 K and a pressure of 4.0 atm
Answers
The volume of 6.9 mol of oxygen at 233 K and a pressure of 4.0 atm is approximately 12.0L.
To calculate volume of a gas, we can make use of Ideal Gas Law equation. It is a fundamental equation in thermodynamics that describes the behaviour of an ideal gas under certain circumstances. It relates pressure(P), volume(V), number of moles (n), and temperature(T) of an ideal gas using the equation:
PV = nRT
Where P = Pressure of the gas,
V = Volume of the gas,
n = Number of moles of the gas,
R = Ideal gas constant commonly expressed as 0.0821 L·atm/(mol·K) or 8.314 J/(mol·K),
T = Temperature of the gas.
In the question, we are given with:
n = 6.9 mol
T = 233 k
P = 4.0 atm
Substituting the above values in the equation to find the volume, we get:
4.0 * V = 6.9 * 0.0821 * 233
V = (6.9 * 0.0821 * 233) / 4.0
V = 11.9997 (approximately 12.0)
Therefore, The volume of 6.9 mol of oxygen at 233 K and a pressure of 4.0 atm is approximately 12.0L.
To study more about Volume of oxygen:
https://brainly.com/question/31630111
https://brainly.com/question/4987534
How many grams of solute would you use to prepare 500mL of 0.25 M magnesium hydroxide solution? with working please, thanks.
Answers
The amount of solute that would be needed to prepare 500 mL of 0.25 M magnesium hydroxide solution will be 7.29 grams.
Stoichiometric problemRecall that: molarity = number of moles of solute/volume of solution
Given a 500 mL solution of magnesium hydroxide with 0.25 M, the number of moles of solute present in the solution can be obtained by:
number of moles = molarity x volume
= 0.25 x 500/1000
= 0.125 mol
Also recall that: number of moles = mass/molar mass. Thus:
mass = number of moles x molar mass.
The molar mass of magnesium hydroxide is 58.32 g/mol.
Thus, mass of 0.125 mol magnesium hydroxide = 0.125 x 58.32
= 7.29 grams.
In other words, 7.29 grams of solute would be used to prepare 500 mL of 0.25 M magnesium hydroxide solution.
More on miscellaneous calculation for solution preparation can be found here: https://brainly.com/question/30096298
#SPJ1
Calculate the mass of ammonium sulfide (NH4)2S in 3.00 L of a 0.0200 M solution.
Answers
The mass of (NH4) 2S in the solution is : Mass = 0.0600 mol × 60.08 g/mol = 3.60 g.
The given molarity and volume of the solution can be used to calculate the number of moles of ammonium sulfide (NH4)2S.Then, the number of moles can be converted to mass using the molar mass of (NH4)2S.Mass of ammonium sulfide (NH4)2S in 3.00 L of a 0.0200 M solution is given by : Mass = moles × molar mass.The number of moles of (NH4)2S can be found using the equation:Molarity = Number of moles / Volume.Rearranging this equation, we get:Number of moles = Molarity × Volume Number of moles of (NH4)2S = 0.0200 M × 3.00 L.Number of moles of (NH4)2S = 0.0600 mol.The molar mass of (NH4)2S can be calculated by summing the molar masses of ammonium (NH4) and sulfide (S) ions.Molar mass of (NH4)2S = (2 × Molar mass of NH4) + Molar mass of S= (2 × 14.01 g/mol) + 32.06 g/mol= 60.08 g/mol.
For more question on mass
https://brainly.com/question/1838164
#SPJ8
what is an isotope
Answers
Answer:
each of two or more forms of the same element that contain equal numbers of protons but different numbers of neutrons in their nuclei, and hence differ in relative atomic mass but not in chemical properties; in particular, a radioactive form of an element.
Explanation:
How moles are in 345.67 liters of water vapor at STP?
Answers
Explanation:
No.of moles =volume in litres/22.4litres
=347.67/22.4=15.52 mole water vapour
Question 1
1 pts
How many grams of sodium is contained in the final container when you dispense 564.2 mL of a
5.72 M sodium chloride solution into a beaker?
The atomic mass of sodium is 22.99 amu
The atomic mass of chlorine is 35.45 amu
Write your answer without units.
Next
Answers
Therefore, there are 73.3 grams of sodium in the final container.
Is sodium chloride acidic or basic?Sodium chloride (NaCl) is a neutral compound, meaning it is neither acidic nor basic. It is a salt formed by the combination of sodium (Na+) and chloride (Cl-) ions, which have a neutral charge and therefore do not affect the pH of a solution.
Firstly, the number of moles of sodium in the solution will be:
n = C * V = 5.72 M * 564.2 mL = 3.21 moles
Next, we convert the number of moles of sodium to grams:
mass = n * atomic mass = 3.21 moles * 22.99 amu/mole = 73.3 grams
To know more about sodium visit :-
brainly.com/question/29327783
#SPJ1
A solution of NaCl was prepared in the following manner: 0.0842 g of NaCl is massed out on an analytical balance. The solid is transferred to a 25.00 mL volumetric flask. Deionized water is added to the flask such that the bottom of the meniscus is at the line. A 1.00 mL aliquot of the stock solution is transferred to a 50.00 mL volumetric flask using a volumetric pipet and diluted to volume. 6. Calculate the concentration of NaCl in the resulting solution in mg/L NaCl. (answer = 67.4 mg/L) 7. Calculate the concentration of NaCl in the resulting solution using propagation of error through the calculation. Use the manufacturer's tolerance values as the absolute error. The tolerances can be found in Chapter 2 of the Harris text. Assume a Class 1 balance and Class A glassware. Treat the tolerances as random error. (answer = 67.4+0.4 mg/L) 8. Identify 2 possible sources of random (indeterminate) error. Identify 2 possible sourses of systematic (determinate) error.
Answers
Two possible sources of systematic (determinate) error in the experiment are; Incorrect calibration of volumetric glasswareIncorrect mass of NaCl
To calculate the concentration of NaCl in the resulting solution in mg/L NaCl, we can use the formula; Concentration (mg/L) = (Mass of solute ÷ Volume of solution in L) × 1000 g / 1 mg NaCl is present in the stock solution of 25 mL. So, the mass of NaCl in the solution would be;0.0842 g ÷ 25 mL = 0.00337 g/mL. Now, in the resulting solution, a 1.00 mL aliquot of the stock solution is transferred to a 50.00 mL volumetric flask and diluted to volume. Therefore, the volume of the resulting solution is 50.00 mL. We will substitute these values in the formula, Concentration (mg/L) = (0.00337 g/mL ÷ 50 mL) × 1000 g / 1 mg concentration (mg/L) = 67.4 mg/L. Therefore, the concentration of NaCl in the resulting solution in mg/L NaCl is 67.4 mg/L.7. Concentration = 67.4 mg/LTolerance = 4.28 mg/LTotal concentration = 67.4 + 4.28 mg/L = 71.68 mg/LWe round off this value to one decimal place; Total concentration = 71.7 mg/LTherefore, the concentration of NaCl in the resulting solution using propagation of error through the calculation is 67.4+0.4 mg/L.8. Two possible sources of random (indeterminate) error in the experiment are; Errors in temperature measurement. Errors in measurement of water volume. Two possible sources of systematic (determinate) error in the experiment are; Incorrect calibration of volumetric glasswareIncorrect mass of NaCl.
Learn more about NaCl
https://brainly.com/question/32275922?
#SPJ11