Answers
they have a negligible mass and they also have a negative charge so they have an association with the magnetic field. Do you remember polar? So electrons are negative and they attract the nucleus which is overall postivie (since protons are positive) so they act as magnetic since they both have an attraction
Related Questions
Which of the following did you include?
The atoms in each ion are bonded together covalently to form a single unit.
The charge is distributed over the entire ion.
The formula of ammonium fluoride is NH4F.
The formula of potassium sulfate is K2SO
Answers
Answer:
The formula of ammonium fluoride is NH4F.
Explanation:
1 The atoms in each ion are bonded together covalently to form a single unit.
Wrong!
⇒ The atoms in an ion compound are linked together by an ionic bond.
2 The charge is distributed over the entire ion.
Wrong!
Ions have taken in or given up electrons. This happens in the atomic shell.
3 The formula of ammonium fluoride is NH4F.
Right!
4 The formula of potassium sulfate is K2SO
Wrong!
The formula for potassium sulfate is K2SO4.
plz help ill give u brainiest
Answers
Answer:
Supercalifragilisticexpialidocious...
Explanation: beacause...so...yeah...
Imagine that scientists have just discovered a non-bird dinosaur skeleton. They want to know whether the dinosaur was closely related to birds. What features in ...
might help them decide?
Answers
Answer:
This evidence includes fossilized bones, teeth, eggs, footprints, teeth marks, and even dung. When paleontologists compare a skeleton of a living bird to the fossilized skeleton of a non-bird theropod, like Sinornithosaurus, they see many similarities.
Explanation:
Answer:
When people think of dinosaurs, two types generally come to mind. There were the huge herbivores,
like Apatosaurus, with their small heads and long tails. There were also those fearsome carnivores,
like Tyrannosaurus rex, that walked on two legs and had a mouthful of teeth like kitchen knives.
Living Dinosaurs
These large dinosaurs are no longer around, but dinosaurs still live among us today. They are the
birds. It's difficult to imagine that a bird on your window sill and a T. rex have anything in common.
One weighs less than a pound. The other was the size of a school bus, tipping the scales at eight
tons. But for all their differences, the two are more similar than you might think. In fact, birds and T.
rex are close relatives. They all belong to a group of dinosaurs called theropods.
This is a cladogram, a "" showing the relationships among organisms. The group called dinosaurs includes the extinct dinosaurs
and all their living descendants. All its members, including living birds, descended from the very first dinosaur-their common ancestor.
That's why birds are a kind of dinosaur (just as humans are a kind of primate).
Skeletal Evidence
When paleontologists compare a skeleton of a living bird to the
fossilized skeleton of a non-bird theropod, like Sinornithosaurus,
they see many similarities. They both have a hole in the hipbone, a
feature that distinguishes most dinosaurs from all other animals.
This feature allows an animal to stand erect, with its legs directly
beneath its body. All theropod dinosaurs, including birds, have a
furcula, also known as a wishbone. Another shared characteristic is the presence of hollow bones.
Hollow bones reduce the weight carried by an animal. This feature enables the animal to run faster. It
probably also played a role in the evolution of flight.
thought to have evolved for flight. The discovery of more and more non-flying dinosaurs with feathers
disproved that explanation. For these dinosaurs, feathers may have served other functions, like
gliding, insulation, protection, and display. Feathers play that same role in many bird species today.
Based on the evidence of shared characteristics, scientists have concluded that birds are a type of
Birds are the only dinosaurs with the ability to fly. This is
very interesting to scientists who want to know when the
capability of flight emerged. To find out, some scientists
study the brains of bird and non-bird dinosaurs. Soft
tissue, such as brains, is almost never preserved in the
fossil record. What is preserved is the imprint the brain
left on the inside of the skull. Now scientists are using
computed tomography (CT) scanners to create
endocasts. These are detailed, three-dimensional
reconstructions of the interiors of fossilized skulls.
In a recent study, researchers were able to peer inside
the braincases of more than two dozen specimens.
"Technology allows us to look inside these specimens
without destroying them," says Dr. Amy Balanoff, a
Museum research associate. "It's a non-destructive way
to basically slice up a dinosaur brain. We look inside and see what it can tell us about the evolution of
the brain within dinosaurs. Most of us grew up thinking that dinosaurs had tiny brains, but actually
some had really big brains."
The endocasts allow Balanoff and other researchers to
explore the outer shape of the brain in more detail. In
addition, the casts also provide new information about
the volume and shape of different regions of the brain.
For example, scientists looked at a detailed view of the
dinosaur cerebrum, a region of the brain related to
cognition and coordination. They found that this region
was very large in non-bird dinosaurs closely related to
birds. Dr. Balanoff's research suggests that these
dinosaurs developed big brains long before flight and that
these bigger brains prepared the way for them to fly.
When examining skeletal, behavioral, and brain
evidence, scientists see that birds and non-bird dinosaurs
share many features. This helped them conclude that
dinosaurs aren't extinct after all. They're living among us today.
(Im a really fast Typer and Thinker)
Have a nice day
What is the PH of sebacic acid ?
Answers
Answer:
Sebacic acid is a naturally occurring dicarboxylic acid with the formula (CH2)8(CO2H)2. It is a white flake or powdered solid. Sebaceus is Latin for tallow candle, sebum is Latin for tallow, and refers to its use in the manufacture of candles.
Explanation:
pls mark as brainliest!!!!!
what are 4 ways a mineral can form
Answers
Answer:
The four main categories of mineral formation are: (1) igneous, or magmatic, in which minerals crystallize from a melt, (2) sedimentary, in which minerals are the result of sedimentation, a process whose raw materials are particles from other rocks that have undergone weathering or erosion, (3) metamorphic, in which new minerals form at the expense of earlier ones owing to the effects of changing—usually increasing—temperature or pressure or both on some existing rock type, and (4) hydrothermal, in which minerals are chemically precipitated from hot solutions within Earth.
The mineral can be formed from volcanic gases, oxidation, crystallization from magma, sediment formation, or deposition from a saline fluid.
What is a mineral?A rock can be described as a collection of minerals. A rock that becomes so hot it melts and many minerals come out in liquids that are hot enough to melt rocks.
Magma can be defined as a melted rock inside Earth, a molten mixture of substances that can be hot to more than 1,000°C. When the magma cools slowly inside the earth, which provides mineral crystals time to grow large enough.
Granite is a rock that produces from slowly cooled magma, consisting of the minerals quartz, plagioclase feldspar which is shiny white, pink potassium feldspar, and black biotite.
When magma will erupt onto the surface of the Earth, it is known as lava. Lava cools more rapidly than magma when it is below the surface and mineral crystals do not have time to form. But the chemical composition remains the same as if the magma cooled slowly.
The mineral can be formed through hydrothermal processes, weathering, and metamorphic and igneous environments.
Learn more about minerals, here:
https://brainly.com/question/1333886
#SPJ2
after 65 minutes, the amount of a particular radioisotope remaining is 3.13% of the initial amount. what is the half life of the radioisotope?
Answers
Answer:
13 mins
Explanation:
We need to find how many half lives are required to reach .0313
.0313 = 1/2^n log both sides and solve for n
log.0313 / log (1/2 ) = n where n= NUMBER of halflives
n = ~ 5 half lives
these 5 half lives took up 65 minutes
so EACH half life is 65/5 = 13 mins
please fast!!!!!!!!!!!!!!!!!!!!!!
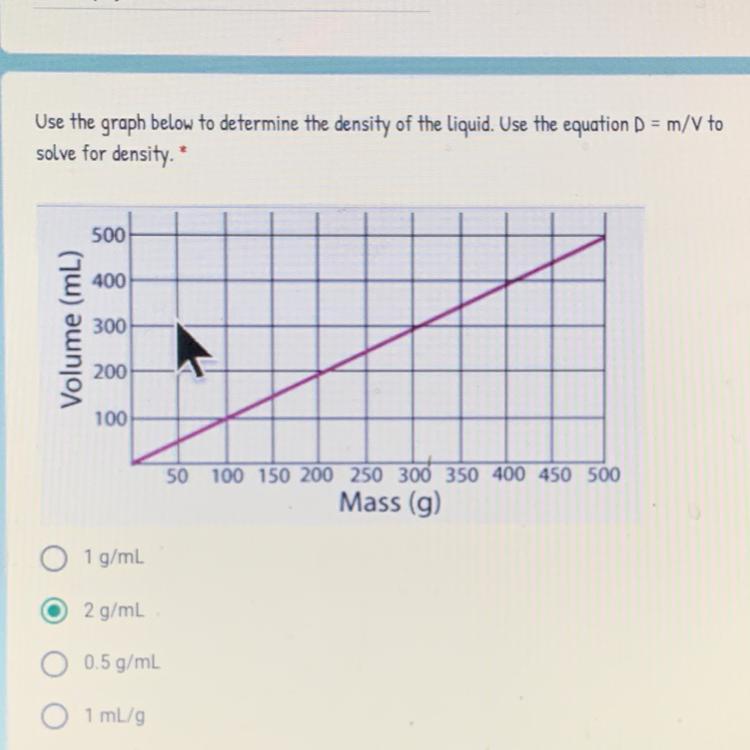
Answers
Answer:
1 g/mL.
Explanation:
From the question given above, the following data were obtained:
Volume = 100 mL
Mass = 100 g
Density =?
Density of a substance is simply defined as the mass of the substance per unit volume of the substance. The density can be expressed mathematically as:
Density (D) = mass (m) / volume (V)
D = m / V
With the above formula, we can obtain the density of the liquid as follow:
Volume (V) = 100 mL
Mass (m) = 100 g
Density (D) =?
D = m / V
D = 100 / 100
D = 1 g/mL
Thus, the density of the liquid is 1 g/mL
gravity sample work connection’s academy
PLEASE HELP AS SOON AS POSSIBLE WILL MARK BRAINIEST
Answers
Answer:
search on YT Khan academy work done by gravity
Explanation:
you will get it for sure with best explanation
mark me please on brainlest
melting point is defined as
Answers
Explanation:
The temperature which a given solid will melt
Answer:
the temperature at which a given solid will melt.
Explanation:
I don't know how to Explain it. (lol)
For a particular reaction at 135.4
°C, Δ=−775.41 kJ/mol
, and Δ=817.91 J/(mol⋅K)
.
Calculate ΔG for this reaction at 12.7
°C.
Answers
Answer:
\(\Delta G=-675.38 \frac{kJ}{mol}\)
Explanation:
Hello!
In this case, for this problem, it is possible to use the thermodynamic definition of the Gibbs free energy:
\(\Delta G=\Delta H-T\Delta S\)
Whereas G, H and S can be assumed as constant over T; thus, we can calculate H at 135.4 °C:
\(\Delta H=\Delta G+T\Delta S\\\\\Delta H=-775.41\frac{kJ}{mol}+(135.4+273.15)K*(0.81791\frac{kJ}{mol*K} )\\\\\Delta H=-441.58\frac{kJ}{mol}\)
Now, we can calculate the Gibbs free energy at 12.7 °C as shown below:
\(\Delta G=-441.58\frac{kJ}{mol} -(12.7+273.15)K*0.81791\frac{kJ}{mol*K}\\\\\Delta G=-675.38 \frac{kJ}{mol}\)
Best regards!
Which of the following elements will form positive ions? Check all
that apply.
Na
z
Cu
Br
Answers
Answer:
cu and Na
Explanation:
When an atom losses electrons this results in a positive charge. A positively charged ion is called a cation.
Sodium and copper will form positive ions. Sodium is a monovalent cation while copper is a divalent cation.
What are positive ions?The ions which contain a positive charge it is called positive ions. The atomic number of sodium is 11. It contains (2, 8, 1) electrons. When it loses it's one electron it will be a positive ion.
Copper has valence electrons. When it loses its two valence electrons it will be a copper ion. There are two types of ions such as positive ions and negative ions.
Small molecules that have acquired a positive charge are known as positive ions. Positive ions are the predominant type of air pollution, including hazardous substances, pollen, mold, pet dander, and other dangerous chemicals.
Therefore, Sodium and copper will form positive ions. Sodium is a monovalent cation while copper is a divalent cation.
To learn more about positive ions, refer to the link:
https://brainly.com/question/269828
#SPJ5
6. How many moles are in 8.30 x 1023 molecules of CO₂?
a.
b.
C.
d.
1.37
2.8
55.5
100
Answers
I need help with the solution

Answers
2. C6H6O2
Answer:
2.) C₆H₆O₂
Explanation:
(Step 1)
To find the molecular formula of hydroquinone, you need to first determine the molar mass of the empirical formula. The molar mass is a sum of the individual atomic weights of each element multiplied by their quantities (denoted by the subscripts).
Atomic Weights:
Carbon (C): 12.011 g/mol
Hydrogen (H): 1.008 g/mol
Oxygen (O): 15.998 g/mol
Molar Mass (C₃H₃O): 3(12.011 g/mol) + 3(1.008 g/mol) + 15.998 g/mol
Molar Mass (C₃H₃O): 55.055 g/mol
(Step 2)
As you can see, the molar mass of the empirical formula is not the same as the molar mass of the molecular formula. Rather, the empirical molar mass is approximately half of the molecular molar mass. Therefore, to make these masses equal, we need to double the empirical formula. This can be done by multiplying all of the subscripts by 2.
55.055 g/mol x 2 = 110.11 g/mol
110.11 g/mol = 110.108 g/mol
C₃ x 2 = C₆
H₃ x 2 = H₆
O x 2 = O₂
Therefore, the correct molecular formula of hydroquinone is C₆H₆O₂.
An aqueous KNO3 solution is made using 75.1 g of KNO3 diluted to a total solution volume of 1.95 L .Calculate the molarity of the solution. (assume a density of 1.05 g/mL for the solution)
Calculate the molality of the solution.
Calculate the mass percent of the solution.
Answers
Answer:
- \(M=0.38M\)
- \(\\ \% m=3.67\%\)
Explanation:
Hello,
In this case, since the molar mass of potassium nitrate is 101.1 g/mol, we can compute the molarity as follows:
\(M=\frac{75.1g*\frac{1mol}{101.1g} }{1.95L} \\\\M=0.38M\)
Moreover, as the mass percent is computed as:
\(\% m=\frac{m_{KNO_3}}{m_{solution}} *100\%\)
Thus, by using the given density of the solution, we obtain:
\(\% m=\frac{75.1g}{1.95L*\frac{1000mL}{1L}*\frac{1.05g}{1mL} } *100\%\\\\ \% m=3.67\%\)
Regards.
The molar heat of fusion for lodine is 16.7 kJ/mol. The specific heat capacity liquid lodine is 0.054 J/g °C.
Calculate the amount of energy (in KJ) required to melt 352 g of solid lodine and then heat the liquid to 180 °C? The
melting point of lodine is 114 °C.
Answer:
Answers
It takes 24.46 kJ of energy to melt 352 g of solid iodine and then heat the resulting liquid to 180°C.
How is the fusion energy's molar value calculated?As the fusion and solidification of a specific substance are completely different processes, the molar heats of fusion and solidification have the same numerical values but opposite signs.
To determine how much energy is needed to melt 352 g of solid iodine, then to heat the resulting liquid to 180°C,
We must first convert the 352 g of iodine we have to moles:352 g / 253.8 g/mol = 1.39 mol
Thus, the energy needed to melt 352 g of solid iodine is as follows:
1.39 mol × 16.7 kJ/mol = 23.2 kJ
The temperature difference is: 180°C - 114°C = 66°C
Thus, the energy needed to raise the liquid iodine's temperature to 180°C is:
352 g × 0.054 J/g°C × 66°C = 1257.8 J = 1.26 kJ
Energy needed to heat liquid iodine plus energy needed to melt solid iodine equals the total amount of energy needed.
= 23.2 kJ + 1.26 kJ
= 24.46 kJ
To know more about solid iodine visit:-
https://brainly.com/question/30575327
#SPJ1
is the quantity of heat needed to
convert a fixed mass of liquid at a fixed
temperature to the gaseous state.
A. Heat of sublimation
B. Heat of pressure
C. Heat of viscosity
D. Heat of vaporization

Answers
If your equation includes 7(CrO4)2, how many Cr's are there?
If your equation includes 7(CrO4)2, how many O's are there?
Answers
If an equation includes 7(CrO₄)₂, the numbers of Cr's and O's atoms that are there are 14 and 56 respectively.
How to calculate number of atoms?The number of atoms present in a chemical compound can be calculated by multiplying the subscript of the particular element by any coefficient.
According to this question, 7 moles of chromate with the chemical formula; (CrO₄)₂ is given. The number of oxygen and chromium atoms in this compound can be calculated as follows:
Chromium = 7 (coefficient) × 2 = 14 atomsOxygen = 7 (coefficient) × 8 = 56 atomsLearn more about no of atoms at: https://brainly.com/question/14190064
#SPJ1
Formation of crystals of sugar from a sugary syrup is a …….
chemical change
Answers
It's a chemical change.
Explanation:-
Formation of crystals of sugar from a sugary syrup is a chemical change. Because, we cannot get sugary syrup back from the sugar crystals. Yet, it is chemical change.
If an insufficient amount of liquid unknown had been used, how would this have effected the value of the experimental molar mass
Answers
Answer:
Actual yield reduces the more.
Explanation:
An actual yield of the course of a chemical reaction is the mass of a product actually obtained from the reaction.
In practice you see it and It is usually less than the theoretical yield.
Various reasons may come up to explain this away but here is one:
• incomplete reactions, simply put here some of the reactants do not react to form the product.
The same applies in the question about the actual yield will reduce significantly in molar mass now that insufficient amount of reagent are used.
For the chemical reaction C2H6 + 137 kJ → C2H4 + H2, the chemical energy of the
a
reactant is greater than the chemical energy of the products.
b
products is greater than the chemical energy of the reactant.
c
reaction is conserved.
d
reactant and the chemical energy of the products are equal.
Answers
For a chemical reaction C₂H₆ + 137 kJ → C₂H₄ + H₂, the chemical energy of the reactant and the chemical energy of the products are equal. Option D is correct.
In an exothermic reaction, energy is released as the reactants are converted to products. The negative sign on the enthalpy change (ΔH) indicates that the reaction is exothermic, meaning that energy is released by the system to the surroundings. This energy comes from the chemical energy of the reactants, and in this case, 137 kJ of energy are released.
The chemical energy of a substance is the energy stored in the bonds between its atoms. In this reaction, C₂H₆ (ethane) is converted to C₂H₄ (ethylene) and H₂ (hydrogen gas), which means that some of the bonds in the reactant molecule are broken and new bonds are formed in the product molecules.
The total amount of energy stored in the bonds of the reactants is equal to the total amount of energy stored in the bonds of the products, since energy cannot be created or destroyed, only converted from one form to another. Therefore, the chemical energy of the reactant and the chemical energy of the products are equal.
Hence, D. is the correct option.
To know more about chemical energy here
https://brainly.com/question/30288262
#SPJ1
what classifies materials as a pure substance or a mixture
Answers
Answer:
A pure substance is a form of matter that has a constant composition and properties that are constant throughout the sample. Mixtures are physical combinations of two or more elements and/or compounds.
Assume you have limp carrot sticks in your refrigerator. To get them plump and crisp again, would you soak them in a hypertonic solution or hypotonic solution?
1) soak them in a hypertonic solution
2) soak them in a hypotonic solution and then the hypertonic solution
3) soak them in a hypotonic solution
(HELP NEED ASAP. THANK U SO MUCH)
Answers
Assume you have limp carrot sticks in your refrigerator. To get them plump and crisp again, we would soak them in a hypotonic solution. So option 3 is correct
What is a hypotonic solution?A hypotonic solution is described as a solution that has lower osmotic pressure than another solution to which it is compared.
A hypotonic solution may also mean a solution that contains a lower amount of solute as compared with the solute concentration in the other solution across a semipermeable membrane.
Since the hypotonic solution has lower osmotic pressure, we will soak the carrots in it to cause crispiness. Due to the cell wall in plant cells, such as carrots, the cells cannot burst from the influx of water, and instead pushes the plasma membrane close to the cell wall, creating “crispiness”.
Learn more about hypotonic solution at: https://brainly.com/question/4237735
#SPJ1
Put these elements in order from most electronegative to least
electronegative.
11 Carbon (C)
11 Nitrogen (N)
11 Fluorine (F)
11 Oxygen (0)
SUBMIT
Need help with putting these in order
Answers
The elements in order from most electronegative to least electronegative is represented as follows:
Fluorine(F)Oxygen(O)Nitrogen(N)Carbon(C)Electronegativity of an atom of elements is the tendency of the atom to attract electron(s) to itself.
Atomic number and the distance of the valency electron from the nuclei affects the electronegativity of an element.
Generally, Non metals are more electronegative than metal. The halogen group are the most electronegative group in the periodic table.
Fluorine is the most electronegative element on earth.
Therefore, the elements in order from most electronegative to least electronegative is represented as follows:
Fluorine(F)Oxygen(O)Nitrogen(N)Carbon(C)learn more on electronegativity here: https://brainly.com/question/11745720?referrer=searchResults
To determine the enthalpy and entropy of dissolving a compound, you need to measure the Ksp at multiple _______. Then, plot ln(Ksp) vs. ______. The slope of the plotted line relates to the _______ of dissolving and the intercept of the plotted line relates to the ______ of dissolving.
Answers
Answer:
To determine the enthalpy and entropy of dissolving a compound, you need to measure the Ksp at multiple temperatures. Then, plot ln(Ksp) vs. 1/T. The slope of the plotted line relates to the enthalpy (ΔH) of dissolving and the intercept of the plotted line relates to the entropy (ΔS) of dissolving.
Explanation:
Hello there!
In this case, according to the given information, it turns out possible for us use the thermodynamic definition of the Gibbs free energy and its relationship with Ksp as follows:
\(\Delta G=-RTln(Ksp)\\\\\Delta G=\Delta H-T\Delta S\)
Thus, by combining them, we obtain:
\(-RTln(Ksp)=\Delta H-T\Delta S\\\\ln(Ksp)=-\frac{\Delta H}{RT} +\frac{T\Delta S}{RT} \\\\ln(Ksp)=-\frac{\Delta H}{RT} +\frac{\Delta S}{R}\)
Which is related to the general line equation:
\(y=mx+b\)
Whereas:
\(y=ln(Ksp)\\\\m=-\frac{\Delta H}{R} \\\\x=\frac{1}{T} \\\\b=\frac{\Delta S}{R}\)
It means that we answer to the blanks as follows:
To determine the enthalpy and entropy of dissolving a compound, you need to measure the Ksp at multiple temperatures. Then, plot ln(Ksp) vs. 1/T. The slope of the plotted line relates to the enthalpy (ΔH) of dissolving and the intercept of the plotted line relates to the entropy (ΔS) of dissolving.
Regards!
1. Elements combine to form millions of
A. metals.
B. mixtures
C. compounds.
2. The forces that hold atoms together in combinations are called
A. energy bonds.
B. nuclear bonds.
C. chemical bonds.
3. Every electron has a
A. neutral charge.
B. positive charge.
C. negative charge.
4. The exact positions of the electrons in an atom cannot be determined because
electrons are always
A. moving
B. sharing.
C. changing
5. Compared with electrons that are closer to the nucleus, those that are farther
away have
A. less energy
B. more energy
C. equal energy
Answers
question no 1 answer is compounds
What is the molarity of a solution of phosphoric acid (a tricrotic acid) if 10.00 mL of the phosphoric acid solution requires 38.11 mL of 0.2412 M NaOH solution to titrate it?
Answers
The phosphoric acid solution has a molarity of 0.3067 M.
How can I determine a solution's molarity?The most common measure for expressing solution concentration is molarity (M), which is calculated by dividing the solute's mass in moles by the volume of the solution in litres: litres of solution/moles of solute equals M.
How is a solution of one molarity made?The term "molarity" (M) refers to the quantity of solute in moles per litre of solution. A clean 1-L volumetric flask should be halfway filled with distilled or deionized water to create a 1 M solution. Slowly add 1 formula weight of the chemical to the flask. Let the compound to completely dissolve, gently turning the flask as needed.
To know more about molarity visit:-
https://brainly.com/question/8732513
#SPJ1
which change of phase is exothermic
Answers
Fusion, vaporization, and sublimation are endothermic processes, whereas freezing,condensation, and deposition are exothermic processes.
How many H atoms are in a 100. mg dose of acalabrutinib, C26H23N7O2?
Answers
There are approximately 0.0105 moles or \(6.3 * 10^20\) hydrogen atoms in a 100. mg dose of acalabrutinib.
To calculate the number of hydrogen atoms in acalabrutinib (\(C_{26} H_{23} N_{7} O_{2}\)), we need to first determine the number of moles of acalabrutinib present in the 100. mg dose, and then use the molecular formula of acalabrutinib to find the number of hydrogen atoms. The molar mass of acalabrutinib can be calculated by adding the atomic masses of its constituent atoms: Molar mass of acalabrutinib = (26 *12.01) + (23 * 1.01) + (7 * 14.01) + (2*16.00) + (7 * 1.01) = 465.51 g/mol. Now we can calculate the number of moles of acalabrutinib in a 100. mg dose: Number of moles = mass ÷ molar mass
= 0.1 g ÷ 465.51 g/mol
= \(2.15 *10^-4 mol\)
Finally, we can use the molecular formula of acalabrutinib to find the number of hydrogen atoms: Number of hydrogen atoms = 23 * (1 H atom per molecule) x (\(2.15 * 10^-4 mol\))
= 0.0105 mol H atoms
To learn more about hydrogen atoms here:
https://brainly.com/question/29695801
#SPJ1
An atomic cation with a charge of +1 has the following electron configuration:
1s 2s 2p 3s 3p 3d ¹4s¹
What is the chemical symbol for the ion?
How many electrons does the ion have?
How many 3p electrons are in the ion?
Answers
A) The chemical symbol for the ion is Fe+
B) It has 20 electrons in total, and there are 6 3p electrons in the ion.
C) There are 6 electrons present in the 3p orbital.
The atomic cation with the given electron configuration is represented by the chemical symbol Fe+.
To determine the number of electrons in the ion, we need to count the electrons present in the electron configuration. In the given configuration, we can see that the 1s orbital has 2 electrons, the 2s orbital has 2 electrons, the 2p orbital has 6 electrons, the 3s orbital has 2 electrons, the 3p orbital has 6 electrons, the 3d orbital has 1 electron, and the 4s orbital has 1 electron. Adding up these numbers, we have:
2 + 2 + 6 + 2 + 6 + 1 + 1 = 20
Therefore, the ion has 20 electrons.
To determine the number of 3p electrons in the ion, we look at the 3p orbital. In this case, there are 6 electrons present in the 3p orbital.
In summary, the chemical symbol for the ion is Fe+, it has 20 electrons in total, and there are 6 3p electrons in the ion.
For more question on electrons
https://brainly.com/question/26084288
#SPJ8
Certain elements are used to make fireworks different colors. Explain how this relates to a line spectrum.
Answers
Answer:
Explanation:
Here, we want to get the relationship between elements used in fireworks and the line spectrum.
The use of fireworks typically involves the application of a source of heat or fire to the fireworks. When this happens, excitation also occurs. The excitation that occurs is also accompanied by a relaxation.
This energy level change would lead to a color change which relies on the energy difference level of the line spectrum and thereby showing the appropriate colour