Cube has a mass of 15g and the volume is 30cm what’s its density
Answers
Related Questions
true or false I need help

Answers
Answer: True
Explanation:
Venus is the hottest planet in the solar system. Mercury is still hot but half of it is also cold due to the sun not directly pointing at it.
Answer:
true
Explanation:
Which image shows a foliated metamorphic rock?
light colored rock with fine grains and metallic luster.
Smooth green rock.
dark color rock with coarse grains in parallel layers.
Answers
Answer:
C
Explanation:
The answer is in the image.
--------------------------------------------------
Walk in faith and trust in God!

WILL GIVE BRAINLIEST!!!
Using 'Why do candles burn?' by Bozeman Science, why is there a change in mass and where does the mass go?
Answers
Answer:
combining wax
Explanation:
Candles burn through an efficient combustion process by combining wax, a poor conductor of heat that melts just above room temperature, with a wick, an absorbent material that will absorb liquid wax and transfer it toward the candles flame while its burning. when a candles wick is lit, the heat from above it. and I'm not a good explainer sorry if I got it wrong
is photosynthesis part of chemical change?If yes can I have photosynthesis in chemical equation as chemical symbols
Answers
Answer:
yes it is a chemical change
i have made it in above picture

Ea for the following uncatalyzed reaction is . Ea for the same reaction when catalyzed is .
(a) What is the ratio of the rate constant for the catalyzed reaction to that for the uncatalyzed reaction at ? Assume that the frequency factor is the same for each reaction
Answers
The ratio of the rate constant for the catalyzed reaction to that for the uncatalyzed reaction can be determined based on the activation energies of the reactions.
What is the ratio of the rate constants?The ratio of the rate constant for the catalyzed reaction (k_cat) to that for the uncatalyzed reaction (k_uncat) can be calculated using the Arrhenius equation:
\(\[ \frac{k_{cat}}{k_{uncat}} = \frac{e^{-\frac{E_{a,cat}}{RT}}}{e^{-\frac{E_{a,uncat}}{RT}}} \]\)
Where Ea,cat is the activation energy for the catalyzed reaction, Ea,uncat is the activation energy for the uncatalyzed reaction, R is the gas constant, and T is the temperature in Kelvin.
Assuming that the frequency factor (A) is the same for both reactions, it cancels out when calculating the ratio. Therefore, the ratio of the rate constants is solely dependent on the activation energies.
Learn more about rate constant
brainly.com/question/20305922
#SPJ11
Which would gain electrons more easily, fluorine or iodine?
Answers
Answer:
i think iodine
Explanation:
Which of these is NOT a reason to record accurate procedures and results during an experiment?
Question 3 options:
So that others can replicate your experiment
So that you can repeat your experiment in the future
Its not that important to record accurate procedures or data
Answers
The option that is NOT a reason to record accurate procedures and results during an experiment is: "Its not that important to record accurate procedures or data."
It is critical to record correct procedures and results so that others may reproduce the experiment and the researcher can repeat it in the future. Proper documentation also aids in the identification of any flaws or inconsistencies that may have happened during the experiment and may be resolved in future studies. Accurate protocol documentation enables other researchers to reproduce the experiment and confirm the findings. Replication is an important part of scientific study since it helps to establish the results' validity and dependability. If the techniques are not adequately documented, subsequent researchers may be unable to replicate the experiment, resulting in differences and doubt regarding the results.
For more such questions on experiment, click on:
https://brainly.com/question/17274244
#SPJ4
GUYS I NEED HELP WITH THIS ASSIGNMENT MY LAST ASSIGNMENT FOR THE DAY
1. What are the reactants? What are the products? Use the reaction below (2pts)
Na + Cl2 → NaCl
2. Why do chemical reactions need to be balanced? (2pt)
3. In order to balance the following reaction, I need to add more Chlorine atoms to the product side. Would the highlighted answer be a correct way of adding more Chlorine atoms? Why or why not? (2pt)
Balanced: Na + Cl2 → NaCl2
4. Given the following reactions, what does the coefficient 2 represent for 2KI? (2pt)
Cl2 + 2 KI → 2 KCl + I2
Answers
Answer:
the reactants are na and cl2 the products is the combination nacl --the law of conservation of matter keeps reactions balanced. No you would not be adding more atoms that is what the first combo is. The coefficient 2 stands for 2 atoms of KL
Explanation:
1 2 3 4 5 6 7 8 9 10 Which point on the number line represents the product (5) (negative 2) (negative 1)? A number line going from negative 11 to positive 11. Point A is negative 10, point B is negative 2, point C is 2, point D is 10. Point A Point B Point C Point D
Answers
Point on the number line represents the product point D
Number line is the pictorial representation of number on straight line called number line
Here given data is a number line in which 10 numbers are seen and this numbers are toward right side it represent positive numbers and toward left side it represent negative numbers so here the point d represent the product and point a represent the reactant so that's why point d which represent 10 are product
Know more about points
https://brainly.com/question/16309605
#SPJ1
4.16 Unit Test: Chemical Bonding - Part 2 1. You are studying alkaline earth metals bonding with Halogens. Using your knowledge of the ionic bonding, what specific and testable scientific question could be asked about the salt formation
Answers
A testable scientific question could be asked about the salt formation is; "Is the salt soluble in water?
A scientific question is a question that can be answered by experiments. In other words, a scientific question must be empirical. Its answer can only be obtained by carrying out experiments.
In the case of bonding in alkaline earth metals, the kind of bond formed is ionic. Ionic compounds are soluble in water. Hence, a testable scientific question could be asked about the salt formation is; "Is the salt soluble in water?
This can be answered by dissolving several salts of alkali earth metals in water.
Learn more: https://brainly.com/question/967776
Convert 5 micrometers to meters.
Answers
Answer:
5e-6
Explanation:
Answer:
0.005
Explanation:
The formula for this equation is dividing the length value by 1000 which will give you your answer which in the case I gave you the answer to your question your welcome. : )
Answer the following questions:
* How is the chemical formula determined for an ionic compound?
* How are the ionic compounds named using IUPAC nomenclature rules?
Answers
Answer:
1. The chemical formula is determined by first finding the positive charge, which is cation, and then finding the negative charge, which is the anion. The cation is writing first, than the anion.
2.An ionic compound is named first by its cation and then by its anion.
Explanation:
Just did it.
one mole of CO2, is equal to how many molecules
Answers
Answer: 1 mole carbon dioxide contains 6.02 x 1023 molecules.
Hope this helps!
How much heat will be absorbed by 320.0 g of water when its temperature is raised by 35.0°C? The specific heat for water is 4.184 J/g°C.
Answers
Answer:
46860.8 joules of heat
Explanation:
Use the formula ΔQ = mcΔT
ΔQ = gain or loss of heat (in joules)
M = mass (in grams),
C = specific heat (in J/g°C)
ΔT = change in temperature (in °C)
Substitute the known values into the formula:
ΔQ = (320.0 g)(4.184 J/g°C)(35.0˚C)
ΔQ = 46860.8 J
q = 320 x 4.184 x 35
so this will give you your answer
poopxpoop equals 1111111111111111111222222222222222233333333333333333333 go
Answers
500 cm3 of a solution has a concentration of 1. 5mol/dm3 how many moles are in the solution?
Answers
Answer:
There is 0.75moles in the solution
Answer:
Explanation:
concentration=no.of moles/volume in dm3
which is
1.5mol/dm3 multiply by 500/1000
=0.75moles
What is an air pressure system?
Answers
Answer:
"The number of air molecules above a surface determines air pressure. As the number of molecules increases, they exert more pressure on a surface, and the total atmospheric pressure increases. By contrast, if the number of molecules decreases, so too does the air pressure."
Show how it’s solve please and thankyou.

Answers
Answer:
160 that is an easy answer
Explanation:
A certain element exists as three different isotopes. 24.1% of all the isotopes have a mass of 75.23 amu, 48.7% have a mass of 74.61 amu, and 27.2% have a mass of 75.20 amu. What is the average atomic mass of this element?
Answers
Answer: A certain element exists as three different isotopes. 24.1% of all the isotopes have a mass of 75.23 amu, 48.7% have a mass of 74.61 amu, and 27.2% have a mass of 75.20 amu. What is the average atomic mass of this element?
Explanation: A certain element exists as three different isotopes. 24.1% of all the isotopes have a mass of 75.23 amu, 48.7% have a mass of 74.61 amu, and 27.2% have a mass of 75.20 amu. What is the average atomic mass of this element? 74.92 amu. Use your periodic table to determine which element this is. As. An element exists
The average atomic mass of the element is 74.93 amu
Let the 1st isotope be A
Let the 2nd isotope be B
Let the 3rd isotope be C
From the question given above, the following data were obtained:
For A (i.e 1st isotope)Abundance of A (A%) = 24.1%
Mass of A = 75.23 amu
For B (i.e 2nd isotope)Abundance of B (B%) = 48.7%
Mass of B = 74.61 amu
For C (i.e 3rd isotope)Abundance of C (C%) = 27.2%
Mass of C = 75.23 amu
Average atomic mass =?The average atomic mass of the element can be obtained as follow:
Atomic mass = [(mass of A × A%)/100] + [(mass of B × B%)/100] + [(mass of C × C%)/100]= [(75.23 × 24.1)/100] + [(74.61 × 48.7)/100] + [(75.23 × 27.2)/100]
= 18.13043 + 36.33507 + 20.46256
Average atomic mass = 74.93 amuThus, the average atomic mass of the element is 74.93 amu
Learn more: https://brainly.com/question/12315958
30 points A 4-column table with 3 rows. The first column titled compound has entries A, B, C. The second column titled melting point (degrees C) with entries negative 183, 660, 772. The third column titled boiling point (degrees C) has entries negative 164, 2467, 1600. The fourth column titled conducts electricity has entries no, yes, yes.
Which compound is most likely a covalent compound?
Answers
The compound that is covalent is the compound A.
Which one is a covalent compound?We know that the forces that holds the molecules of an ionic substance together is so strong that the ionic substances are known to have a high melting and a high boiling point. Thus, it is already clear that the compounds B and C are both ionic because they are highly conducting and they also have a high melting and boiling point.
The compound A has a low melting and boiling point and also does not conduct electricity. This goes to tell us that A is a covalent compound.
Learn more about covalent compound:https://brainly.com/question/11632372
#SPJ1
Answer:
compound A
Explanation:
edge 2023
a 0.135 g sample of a monoprotic acid of unknown molar mass is dissolved in water and titrated with 0.1003 m naoh. the equivalence point is reached after adding 21.36 ml of base.
Part A
What is the molar mass of the unknown acid?
Molar mass = g/mol
Answers
The molar mass of the unknown acid is 150 g/mol.
Mass of acid (m) = 0.135 g
Volume of NaOH = 21.36 mL = 0.02136 L
Concentration of NaOH (c) = 0.1003 M
The balanced equation is:Acid + NaOH → NaSalt + Water
Molar mass of the unknown acid can be calculated by using the formula;molar mass of acid = (mass of acid used × molar mass of NaOH × volume of NaOH used) / (number of hydrogen ions × 1000)
Mass of NaOH = Concentration × volume = 0.1003 × 0.02136 = 0.002145 mol
Mass of acid used = 0.135 g
Molar mass of NaOH = 40 g/mol
Number of hydrogen ion = 1
Volume of NaOH used = 21.36 mL = 21.36/1000 = 0.02136 L
Molar mass of acid = (0.135 × 40 × 0.002145) / (1 × 0.02136)
Molar mass of acid = 149.97 g/mol ≈ 150 g/mol
Therefore, the molar mass of the unknown acid is 150 g/mol.
Learn more about molar mass with the given link,
https://brainly.com/question/837939
#SPJ11
a student was given the task of titrating a 20.ml sample of 0.10mhcl(aq) with 0.10mnaoh(aq). the hcl(aq) was placed in an erlenmeyer flask along with two drops of an appropriate acid-base indicator. an equation for the reaction that occurs during the titration is given above. (a) according to the equation for the reaction, if the number of moles of reactants is halved, how does this affect the number of moles of h2o(l) produced in the reaction?
Answers
According to the equation for the reaction, if the number of moles of reactants is halved, the number of moles of H₂O (l) produced in the reaction is halved as well.
What is a titration?A titration is a procedure in volumetric analysis in which a given volume of a solution of a known concentration is added to a given volume of a solution of an unknown concentration to determine the unknown concentration.
The moles of the substances that reacted as given in the equation of the reaction are used to determine the unknown concentration.
The equation of the given reaction is given below:
HCl + NaOH ---> NaCl + H₂O
1 mole of HCl reacts with 1 mole of NaOH to form 1 mole of water.
Hence, halving the moles of reactants would halve the mole of water formed.
Learn more about titration at: https://brainly.com/question/15170788
#SPJ1
How many grams of solid LiCl are needed to make 250. ml of a 0.125 m solution?
Answers
1.325 g of solid LiCl is needed to make 250 ml of a 0.125 m solution.
How do you calculate the mass of solid LiCl are needed to make 250. ml of a 0.125 m solution?We can use the following formula to determine how many grams of LiCl are needed to create a 0.125 m solution:
Molarity (M) is calculated as moles of solute per liter of solution.
Molarity (M) x liters of solution equals moles of solute.
Inputting the values provided yields:
LiCl moles are equal to 0.125 mol/L times 0.250 L, or 0.03125 mol.
We must utilize the molar mass of LiCl, which is 42.39 g/mol, to convert moles of LiCl to grams. LiCl is therefore needed in the following mass:
LiCl's mass is calculated by multiplying its molar mass (42.39 g/mol) by its molecular weight (0.03125 mol). The result is 1.325 g of LiCl.
Hence, to make 250 mL of a 0.125 m solution we require 1.325 g of solid LiCl.
To learn more about molarity visit:
brainly.com/question/8732513
#SPJ1
(a) Barium ions are poisonous. Patients with digestive tract problems are sometimes given
an X-ray after they have swallowed a ‘barium meal’, consisting of a suspension of
BaSO4 in water. The [Ba2+(aq)] in a saturated solution of BaSO4 is too low to cause
problems of toxicity.
(i) Write an expression for the solubility product, Ksp, for BaSO4, including its units.
...................................................................................................................................
(ii) The numerical value of Ksp is 1.30 × 10–10. Calculate [Ba2+(aq)] in a saturated
solution of BaSO4.
...................................................................................................................................
...................................................................................................................................
(iii) The numerical value of Ksp for BaCO3 (5 × 10–10) is not significantly higher than
that for BaSO4, but barium carbonate is very poisonous if ingested. Suggest a
reason why this might be so.
...................................................................................................................................
............................................................................
QUESTION NUMBER (b)(iii) and (ii) PLEASE....
Answers
The numerical value of the Ksp of \(BaSO_{4}\) is 1.69 * 10^-20.
What is the Ksp?The Ksp is an equilibrium constant that shows the extent to which a substance is soluble in water. Now consider the fact that \(BaSO_{4}\) is almost insoluble in water.
i) The Ksp of the \(BaSO_{4}\) solution can be obtained from the relation;
Ksp = [\(Ba^{2+}\)] [\(SO_{4}^{2-}\)]
ii) The numerical value of the Ksp is obtained from; [1.30 × 10–10]^2 = 1.69 * 10^-20
iii) The reason for the toxicity of \(BaCO_{3}\) even though it is not more soluble that barium sulfate is that \(BaCO_{3}\) can dissolve in the gastrointestinal tract which is acidic leading to barium poisoning.
Learn more about barium poisoning:https://brainly.com/question/14593131
#SPJ1
PLS HELP ASAP 15 POINTS TYSM which of the following is a consequence of urban heat islands? a) increased precipitation downwind of the city b) increased winds within the city c) increased precipitation upwind of the city d) decreased winds within the city
Answers
Answer:
Hey!
Your answer is definitely A!
Explanation:
Hope this helps!
:>
In an ecosystem, the following is a consequence of urban heat islands which is increased precipitation downwind of the city.
What is an ecosystem?Ecosystem is defined as a system which consists of all living organisms and the physical components with which the living beings interact. The abiotic and biotic components are linked to each other through nutrient cycles and flow of energy.
Energy enters the system through the process of photosynthesis .Animals play an important role in transfer of energy as they feed on each other.As a result of this transfer of matter and energy takes place through the system .Living organisms also influence the quantity of biomass present.By decomposition of dead plants and animals by microbes nutrients are released back in to the soil.
Learn more about ecosystem,here:
https://brainly.com/question/1673533
#SPJ2
The horses on a carousel or merry-go-round are accelerating because
Answers
A car starts from rest and reaches a top speed of 80 m/s. If the car did this is 20 seconds . What is the acceleration ?
Answers
somethingExplanation:
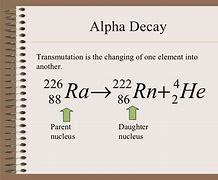
226 • Ra 4/2 He + 88 = ?
Answers
Answer: the answer for this question is in the pic
Explanation:
. Sketch the nucleotide being described: it uses a monosaccharide present in RNA, and a nitrogenous base found only in RNA. Point an arrow to the glycosidic bond.
Answers
Answer:
See below
Explanation:
The glycosidic bond forms between the carbon atom on the glucose molecule and the nitrogen atom present on the nitrogenous base. A diagram has been attached to show this particular bond. There is also a phosphate molecule bonded on the sugar molecule at the other end.

Determine the number of atoms of O in 32.3 moles of Al₂(SO₄)₃.
Answers
387.6 number of atoms of oxygen are in 32.3 moles of Al₂(SO₄)₃.
What do you mean by the mole concept ?
Mole is the amount of substance in a chemical system that contains as many elementary entities as there are atoms in exactly 12 grams of the carbon−12 isotope.
The mole concept is a convenient method of expressing the amount of a substance.
To calculate the the number of atoms of O in 32.3 moles of Al₂(SO₄)₃-:
The chemical formula for aluminum carbonate, Al₂(SO₄)₃ , indicates that in one mole of the compound there are twelve moles of oxygen atoms.
Now,
=22.3 m o l Al₂(SO₄)₃× 12 m o l O atoms/1 m o l Al₂(SO₄)₃
= 387.6 m o l of O atoms
Hence, 387.6 number of atoms of oxygen are in 32.3 moles of Al₂(SO₄)₃.
Learn more about mole concept ,here:
https://brainly.com/question/22540912
#SPJ1