Calculate the mass of a liquid with a density of 0.92g/ml and a volume of 15 ml
Answers
Answer:
13.8g
Explanation:
Remember that density is equal to mass over volume
d=m/v
rearrange the problem and you get dv=m
(0.92g/mL)(15mL)=13.8g
Related Questions
write down three use of chlorine
Answers
Answer:
Chlorine is commonly used as an antiseptic and is used to make drinking water safe and to treat swimming pools. Large amounts of chlorine are used in many industrial processes, such as in the production of paper products, plastics, dyes, textiles, medicines, antiseptics, insecticides, solvents and paints.
Explanation:
hope this helped you
please mark as the brainliest (ㆁωㆁ)
1What are 2 examples of proteins in the body?
Answers
Answer:
1. Insulin
2. Keratin
Explanation:
Proteins are one of the four biological molecules found in living systems. They are polymeric molecules made up of monomeric units called AMINO ACIDS. Proteins perform a wide variety of functions in the body ranging from enzymatic functions to structural roles.
Two examples of proteins found in the body are keratin, insulin. Keratin is a structural protein located in the skin, hair and nails while insulin is an enzymatic protein that aids in digestion.
Molarity of Kool Aid solutions can be calculated by comparing the concentrations of Kool Aid powder and sugar added to a given volume of water. The molar mass of Kool Aid will be the same as that of sugar for our purpose. The molecular formula for sugar is C12H22O11- Your objective for this lab will be to calculate the molarity of Kool Aid desired based on package directions. You will then be provided two concentrated Kool Aid solutions. You will use dilution calculations to determine the amount of water and concentrated solution you will need in order to prepare 65 mL of the desired molarity.
Calculate the molarity of Kool Aid desired based on the following information from the package directions.
1 package Kool Aid powder = 4. 25 grams 1 cup sugar = 192. 00 grams
2. 00 quarts of water (1. 06 quarts = 1 liter)
Answers
The amount of concentrated solution needed is (0.286 M)(65 mL) / C M, and the amount of water needed is 65 mL minus the volume of the concentrated solution.
To calculate the molarity of Kool Aid desired, we need to determine the number of moles of Kool Aid powder and sugar in the package. Since the molecular formula for sugar is C12H22O11, we can calculate its molar mass as follows:
Molar mass of C12H22O11 = (12 * 12.01) + (22 * 1.01) + (11 * 16.00)
= 144.12 + 22.22 + 176.00
= 342.34 g/mol
Given that the package contains 4.25 grams of Kool Aid powder, we can calculate the number of moles of Kool Aid powder using its molar mass:
Number of moles of Kool Aid powder = Mass / Molar mass
= 4.25 g / 342.34 g/mol
≈ 0.0124 mol
Similarly, for the sugar, which has a molar mass of 342.34 g/mol, we can calculate the number of moles of sugar using its mass:
Number of moles of sugar = Mass / Molar mass
= 192.00 g / 342.34 g/mol
≈ 0.5612 mol
Now, to calculate the molarity of the desired Kool Aid solution, we need to determine the volume of water. Given that 1.06 quarts is equal to 1 liter, and we have 2.00 quarts of water, we can convert it to liters as follows:
Volume of water = 2.00 quarts * (1.06 liters / 1 quart)
= 2.12 liters
To find the molarity, we use the formula:
Molarity (M) = Number of moles / Volume (in liters)
Molarity of Kool Aid desired = (0.0124 mol + 0.5612 mol) / 2.12 L
≈ 0.286 M
To prepare 65 mL of the desired molarity, we can use dilution calculations. We need to determine the volume of concentrated solution and the volume of water needed.
Let's assume the concentration of the concentrated Kool Aid solution is C M. Using the dilution formula:
(C1)(V1) = (C2)(V2)where C1 is the initial concentration, V1 is the initial volume, C2 is the final concentration, and V2 is the final volume.
Given that C1 = C M and V1 = V mL, and we want to prepare a final volume of 65 mL (V2 = 65 mL) with a final concentration of 0.286 M (C2 = 0.286 M), we can rearrange the formula to solve for the volume of the concentrated solution:
(C M)(V mL) = (0.286 M)(65 mL)
V mL = (0.286 M)(65 mL) / C M
So, the amount of concentrated solution needed is (0.286 M)(65 mL) / C M, and the amount of water needed is 65 mL minus the volume of the concentrated solution.
For more such questions on concentrated visit:
https://brainly.com/question/28564792
#SPJ8
define each of the terms below IN YOUR OWN WORDS
Environmental Value System:
Entropy:
Equilibrium:
Feedback:
Feedback, negative:
Feedback, positive:
Gaia:
Model:
Answers
Answer:
1- Environmental Value System or EVS is a worldview that molds the way both individuals and the societies they form perceive and therefore evaluate environmental concerns.
2-Entropy/ the measure of a system's thermal energy per unit temperature that is unavailable for doing useful work.
3-Equilibrium/a state of balance between opposing forces or actions that is either static
4-Feedback/a return of information about a result or the returned portion of a process.
5-Feedback,negative/ is a self-regulatory system in which it feeds back to the input a part of a system's output so as to reverse the direction of change of the output.
6-Feedback,positive/ is the amplification of a body's response to a stimulus.
7-Gaia/ all organisms and their inorganic surroundings on Earth are closely integrated to form a single and self-regulating complex system
8-Model/a physical and/or mathematical and/or conceptual representation of a system of ideas, events or processes.
2. If you start with 20.3 g of NH_NO3, how many grams of O, will be produced? Nuore also no
Answers
Answer:
12.18g
Explanation:
You start with a mass of 20.3g of NH₄NO₃
We need to determine from this compound the mass of oxygen that is therein.
First, find the molar mass of the compound;
NH₄NO₃ = 14 + 4(1) + 14 + 3(16) = 80g/mol
So;
Mass of oxygen = \(\frac{48}{80}\) x 20.3 = 12.18g
Since in a chemical reaction, mass is always conserved, 12.18g of oxygen is produced.
how many g are in 16822 mg?
Answers
Answer:
it is jues
Explanation:
1 know
The correct placement of the coefficient in a chemical equation is...
Select one:
a. within the formula of a reactant or product.
O b. in front of the formula of a reactant or product.
O c. in front of the chemical equation.
O d. after the formula of a reactant or product.
Answers
Answer:
B.) in front of the formula of a reactant or product.
Explanation:
Coefficients modify the amount of a particular molecule. As such, they are placed directly in front of the molecule's formula.
You are given 52 grams of water. What volume container do you need to hold all the water? (Density of water = 1.0 g/mL)
Answers
Answer:
52 ml water is present so we need any container more than 52 ml
Explanation:
Density = mass / volume
Volume= mass/ density
52/1= 52 ml
When someone opens the lunch box we get smell. Why?
Answers
Answer:
Diffusion
Explanation:
When someone opens a lunch box, the molecules of the food inside it start to diffuse into the surrounding air. Diffusion is the process by which molecules move from an area of higher concentration to an area of lower concentration. In this case, the molecules of the food are at a higher concentration inside the lunch box, and when the box is opened, they start to spread out into the air where their concentration is lower.
As the molecules of the food move out of the lunch box and into the air, they collide with the air molecules, and this causes them to spread out even further. The movement of the food molecules and their collision with the air molecules creates an odor that we can smell. This odor is actually made up of the molecules of the food that have diffused into the air.
The diffusion of the food molecules into the air is a natural process that occurs because of the difference in concentration between the food and the air. This diffusion continues until the concentration of the food molecules in the air reaches equilibrium with the concentration of the food molecules inside the lunch box. At this point, the odor will no longer be noticeable because the concentration of the food molecules in the air will be the same as the concentration of the food molecules inside the lunch box.
How many moles are in 9.62 x 1023 atoms of Iron (Fe)?
Show work!!
Answers
Answer:
To determine the number of moles in 9.62 x 10^23 atoms of Iron (Fe), we need to use Avogadro's number, which is 6.022 x 10^23 particles per mole.
First, we need to divide the number of atoms by Avogadro's number to get the number of moles:
9.62 x 10^23 atoms / 6.022 x 10^23 atoms/mol = 1.599 moles of Iron (Fe)
For many plants, carbon dioxide is a limiting factor.
What happens when more carbon dioxide is available to plants?
a.Plant growth increases.
b.Plant growth stays the same.
c.Plant growth decreases.
d.Plant growth may increase or decrease.
Answers
The correct answer is option a
How does increased carbon dioxide availability affect plant growth?Increased availability of carbon dioxide has a direct impact on plant growth. Carbon dioxide is a critical component of photosynthesis, the process through which plants convert light energy into chemical energy. In normal conditions, carbon dioxide concentrations in the atmosphere can be a limiting factor for photosynthesis.
When more carbon dioxide is available, plants are able to take in higher amounts of this gas, leading to increased rates of photosynthesis. As a result, plants experience enhanced growth, including increased biomass, larger leaves, and improved reproductive capacity.
Carbon dioxide, along with water and sunlight, is essential for photosynthesis. Higher carbon dioxide levels can potentially stimulate photosynthesis and have been observed to improve plant productivity in certain environments.
Learn more about:Increased availability of carbon dioxide
brainly.com/question/20513967
#SPJ11
What is called exothermic
Answers
An exothermic process is one that gives off heat.
Explanation:
This heat is transferred to the surroundings. An endothermic process is one in which heat has to be supplied to the system from the surroundings.
What compound is formed from Ca2+ and Cl1-
Answers
Answer:
You get CaCl2 (Calcium chloride)
Explanation:
Calcium has a +2 charge and chlorine has a -1 charge. You need the net ionic charge to equal zero so you need two chlorine atoms to have the +2 cancel out. You end up with CaCl2.
what combination of carbonyl compounds would react to form the following product?
Answers
The desired product can be obtained by reacting a ketone with a primary amine in the presence of a reducing agent, such as sodium cyanoborohydride. This reaction is known as reductive amination.
The desired product can be synthesized through a reductive amination reaction, which involves the condensation of a carbonyl compound with a primary amine followed by reduction. In this case, a ketone is required as the carbonyl compound.
The first step involves the condensation of the ketone with the primary amine. The carbonyl group of the ketone reacts with the amine group of the primary amine, forming an imine intermediate. This condensation reaction is typically catalyzed by an acid, such as hydrochloric acid or sulfuric acid. The imine intermediate is formed as an imine linkage between the carbon of the carbonyl group and the nitrogen of the amine group.
The second step is the reduction of the imine intermediate to the desired product. This reduction is achieved by using a reducing agent, such as sodium cyanoborohydride (NaBH3CN). The reducing agent donates a hydride ion (H-) to the imine, resulting in the formation of the desired product, which is an amine.
Learn more about reductive amination :
https://brainly.com/question/14207331
#SPJ11
Answer:
Carbonyl compounds which are of low molecular weight (organic acids, ketones, and aldehydes) can undergo carbon coupling reactions to produce gasoline and diesel.
10.
Given the following reaction
Ca(s) + 2HCl(aq) → CaCl2(aq) + H2(g)
If 1.00 g of Ca reacts with 1.00 g HCI and 1.21 g CaCl2 are produced, what is the theoretical yield, the percent
yield and the limiting reactant?
a. 2.77 g, 43.7%, Ca limiting
b. 2.77 g, 79.5%, HCI limiting
c. 1.52 g, 43.7%, Ca limiting
d. 1.52 g, 79.5% HCI limiting
e. 2.77g, 54.9%, Ca limiting
f. 1.52 g, 54.9%, HCI limiting
Answers
The given reaction is calcium, solid, plus 2 h c, l gives c a c l, 2 plus hydrogen. Here we can write 1 mole of calcium, reacting with 2 moles of h, c l ...
Given the following reaction Ca(s) + 2HCl(aq) → CaCl2(aq) + H2(g) If 1.00 g of Ca reacts with 1.00 g HCI and 1.21 g CaCl2 are produced,
the mass of ice that can be melted by 1 gram of 100-degree-c steam is(hint: don't forget about hot water remaining from condensed steam.)question 46 options:0.148 gram.0. 125 gram.8 grams.6.75 grams.
Answers
The mass of ice that can be melted by 1 gram of 100°C steam is 6.75 grams. The result is obtained by using the Black's Principle.
What is the Black's Principle?The Black's Principle states that heat released by a higher-temperature object is equal to the heat absorbed by a lower-temperature object. It can be expressed as
Q release = Q absorbs
Where
Q release is the heat released by high-temperature objectsQ absorb is the heat absorbed by low-temperature objectsGiven
The mass of steam, m₁ = 1 gramThe temperature of steam, T₁ = 100°CWhat is the mass of ice that can be melted if it is mixed with the steam?
If they are mixed, the steam will release the heat and the ice will absorb the heat. Remember that:
heat of vaporization of water, Lv = 540 kal/gheat of fusion of water, Lf = 80 kal/gQ release = Q absorbs
m₁ Lv = m₂ Lf
1 × 540 = m₂ × 80
m₂ = 540 ÷ 80
m₂ = 6.75 gram
Hence, the mass of ice that can be melted is 6.75 gram.
Learn more about Black's Principle here:
brainly.com/question/14142460
#SPJ4
a compound is found to contain 53.70% iron and 46.30% sulfur. Find The Empirical Formula Of A Compound Containing 53.70% Iron And 46.30% Sulfur
Answers
Fe2S3 is The Empirical Formula Of A Compound Containing 53.70% Iron And 46.30% Sulfur.
Fe = 0.961 mol and S = 1.44 mol, Now divide them to get lowest empirical formula. When the subscripts in a chemical formula represent the simplest ratio of the kinds of atoms in the compound, the formula is called an empirical formula.
Most ionic compounds are described with empirical formulas. A molecular formula describes the actual numbers of atoms of each element in a molecule. A compound that contains ions and is held together by ionic bonds is called an ionic compound. The periodic table can help us recognize many of the compounds that are ionic:
When a metal is combined with one or more nonmetals, the compound is usually ionic. This guideline works well for predicting ionic compound formation for most of the compounds typically encountered in an introductory chemistry course. However, it is not always true (for example, aluminum chloride, AlCl3, is not ionic).
To know more about empirical formula here
https://brainly.com/question/1439914
#SPJ4
Draw the alkyl bromide(s) you would use in a malonic ester synthesis of ethyl 2-methyl-4-pentenoate
Answers
The structure of the alkyl bromides used in a malonic ester synthesis of ethyl 2-methyl-4-pentenoate are as drawn in the attached image.
Ethyl 2-methyl-4-pentenoate by Malonic ester synthesis.The malonic ester synthesis is a chemical reaction characterized by the alkylation of diethyl malonate or a similar ester of malonic acid at the carbon alpha (directly adjacent) to both carbonyl groups, and subsequently converted to a substituted acetic acid.
Hence, it follows from the structure of Ethyl 2-methyl-4-pentenoate that the alkyl bromides used are Ethyl bromide and methyl bromide.
Read more on Malonic ester synthesis;
https://brainly.com/question/17237043
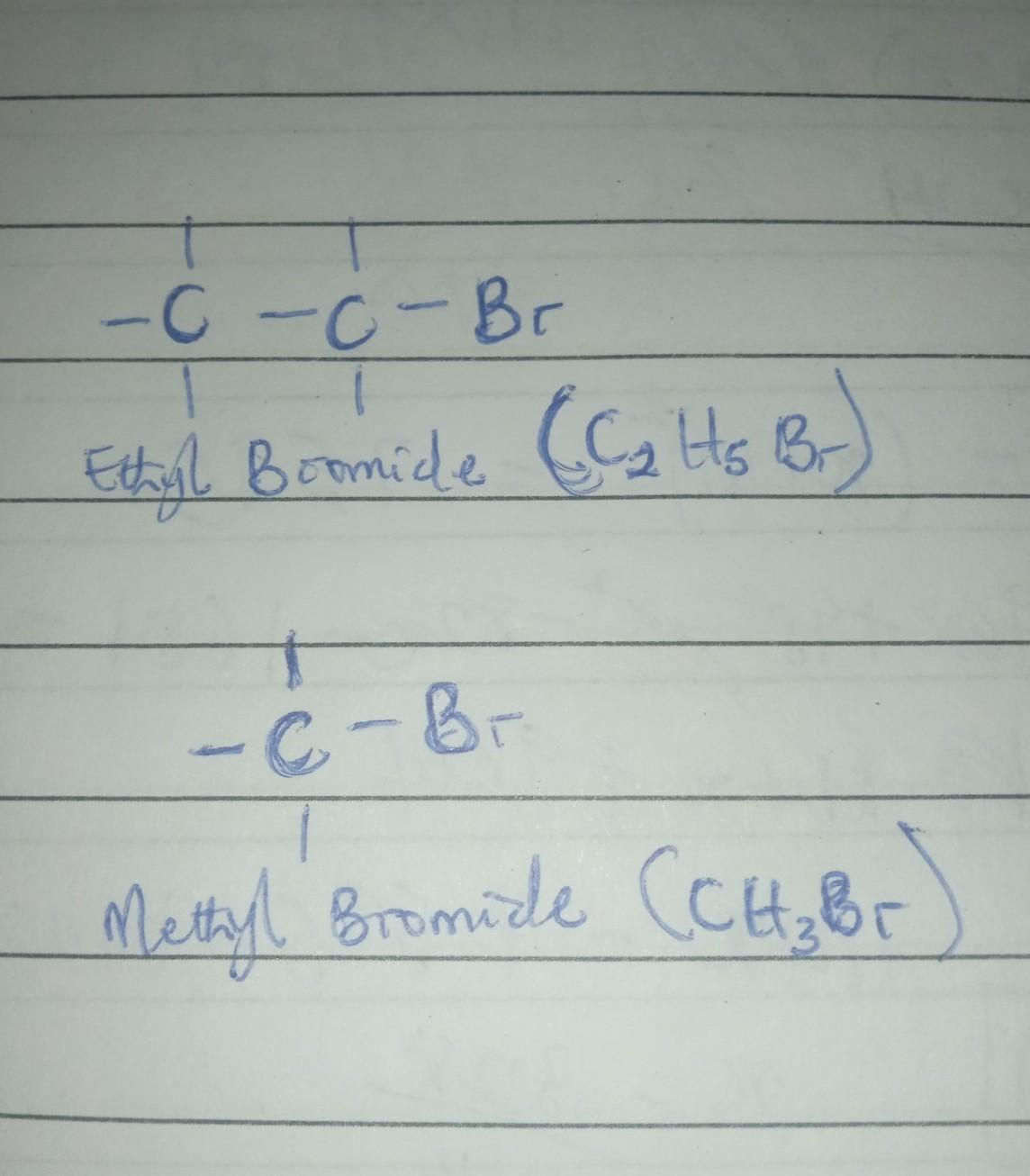
The structure of alkyl bromide that would be used in a malonic ester synthesis of ethyl 2-methyl-4-pentenoate are ethyl bromide and methyl bromide.
What is alkyl bromide?
When halogens such as Br, Cl, I, attched to the sp3 carbon atom of alkyl group, they called alkyl halides.
Here, bromide is attached to an alkyl group.
Ethyl bromide and methyl bromide are the alkyl halides used in synthesis of ethyl 2-methyl-4-pentenoate
Thus, ethyl bromide and methyl bromide are the alkyl bromides.
Learn more about alkyl bromide
https://brainly.com/question/14126879
#SPJ4


BRAINLIEST IF RIGHTLet’s imagine now that you are in a spaceship in outer space, far from any stars or planets, where there is no air. If you fire the rocket engine, an unbalanced force is created that propels your spaceship forward.
What will happen when you turn off the rocket engine?
Group of answer choices
The rocket's velocity will decrease over time and distance.
The rocket will stop immediately.
The rocket will continue to travel at a constant velocity.
The rocket's velocity will increase with time and distance.
Answers
Answer: b
Explanation:
Answer:
C
Explanation:
Obviously, as there is no air to create friction or any opposing force against the rocket; and there is no force accelerating it.
The rocket will maintain its velocity
How many particles are there in 65 grams of potassium iodide (KI)?
Answers
Answer:
0.3915597312020745
Explanation:
Answer:
Look Down :)
Explanation:
To determine the number of particles in 65 grams of potassium iodide (KI), we need to use the Avogadro's constant (NA) and the molar mass of KI.
The molar mass of KI can be calculated by adding the atomic masses of potassium (K) and iodine (I) in one KI molecule. The atomic masses of K and I are 39.10 g/mol and 126.90 g/mol, respectively. Therefore, the molar mass of KI is:
Molar mass of KI = atomic mass of K + atomic mass of I
= 39.10 g/mol + 126.90 g/mol
= 166.00 g/mol
Now we can calculate the number of particles in 65 grams of KI using the following formula:
Number of particles = (mass of sample / molar mass) x Avogadro's constant
Number of particles = (65 g / 166.00 g/mol) x 6.022 x 10^23 particles/mol
= 2.51 x 10^23 particles
Therefore, there are approximately 2.51 x 10^23 particles in 65 grams of potassium iodide (KI).
Atmospheric greenhouse gases Group of answer choices absorb longwave radiation but do not impede the transfer of latent and sensible heat absorb shortwave radiation and also impede the transfer of latent and sensible heat absorb shortwave radiation but do not impede the transfer of latent and sensible heat absorb longwave radiation and also impede the transfer of latent and sensible heat
Answers
Atmospheric greenhouse gases absorb longwave radiation but do not impede the transfer of latent and sensible heat. Carbon dioxide (CO2), methane (CH4), and water vapor are examples of glasshouse gases that may absorb longwave radiation (infrared radiation) generated by the Earth's surface. Hence option A is correct.
The glasshouse effect and the planet's warming are caused by glasshouse gases absorbing longwave radiation. Yet, the movement of latent and sensible heat is unaffected by glasshouse gases.
The energy absorbed or released during phase shifts, such as evaporation and condensation, is referred to as latent heat. The energy that is exchanged between substances or objects without a phase shift is referred to as sensible heat. Via these methods, glasshouse gases do not obstruct the passage of heat.
To know more about greenhouse gases:
https://brainly.com/question/28138345
#SPJ4
For a molecular compound of formula EFA (where E is an element), match the electron-pair geometry with the element. Trigonal bipyramidal I Tetrahedral Si Octahedral
Answers
The electron-pair geometry that matches the molecular compound of formula EFA (where E is an element) with the element Tetrahedral is Si.
In the case of the molecular compound of formula EFA (where E is an element), the electron-pair geometry that matches the element Tetrahedral is Si. This is because the compound SiF4 follows the tetrahedral geometry of electron pairs. The four fluorine atoms present around silicon form the corners of a tetrahedron. This results in the tetrahedral geometry of SiF4.
The tetrahedral geometry is a molecular geometry that is present when there are 4 bonds and 0 lone pairs around the central atom. In other words, the central atom is bonded with four other atoms or groups. The tetrahedral geometry is also one of the most common geometries found in the study of Chemistry.
Learn more about electron-pair geometry
brainly.com/question/30901122
#SPJ11
1. How many grams are in 1.7 x 10^23 particles of Cl2?
2. How many moles are in 3.28 x 10^23 atoms of NaCl? *
3. If I were to determine how many liters 26 grams of water is, what type of conversion would this be? *
A Mass --> Moles --> Particles
B Mass --> Moles --> Volume
C Volume --> Mass --> Moles
D Moles --> Mass --> Volume
Answers
Answer: 1. 20.0 grams
2. 0.272 moles
3. B) Mass --> Moles --> Volume
Explanation:
According to avogadro's law, 1 mole of every substance weighs equal to molecular mass and contains avogadro's number \(6.023\times 10^{23}\) of particles.
To calculate the number of moles, we use the equation:
\(\text{Number of moles}=\frac{\text{Given molecules}}{\text{Avogadros number}}\) or
\(\text{Number of moles}=\frac{\text{Given mass}}{\text{Molar mass}}\) or
Putting in the values we get:
1. \(\text{Number of moles of} Cl_2=\frac{1.7\times 10^{23}}{6.023\times 10^{23}}=0.282moles\)
Mass of \(Cl_2=moles\times {\text {Molar mass}}=0.282mol\times 71g/mol=20.0g\)
2. \(\text{Number of moles of NaCl}=\frac{3.28\times 10^{23}}{2\times 6.023\times 10^{23}}=0.272moles\)
3. \(\text{Number of moles of water}=\frac{26g}{18g/mol}=1.44moles\)
Volume of water =\(moles\times {\text {Molar volume}}=1.44mol\times 22.4L/mol=32.4L\)
Compare and contrast the Ionization energies of groups 1&2 (Alkali and Alkaline metals) with the Ionization energies of groups 16&17 (Oxygen family and Halogens)
Answers
Answer:
Ionization energy is the energy required to remove an electron from a specific atom. It is measured in kJ/mol, which is an energy unit, much like calories. The ionization energies associated with some elements are described in the Table 1. For any given atom, the outermost valence electrons will have lower ionization energies than the inner-shell kernel electrons. As more electrons are added to a nucleus, the outer electrons become shielded from the nucleus by the inner shell electrons. This is called electron shielding .
Explanation:
a little summary
Ionization energy refers to the amount of energy needed to remove an electron from an atom.
Ionization energy decreases as we go down a group.
Ionization energy increases from left to right across the periodic table.
How will the rate of the reaction change if the concentration of H3CNH2 is tripled? A. The rate will decrease by a factor of 3. B. The rate will not change. C. The rate will increase by a factor of 3. D. No conclusion can be made based on the presented information.
Answers
The correct option is C. The rate of the reaction change if the concentration of H3CNH2 is tripled The rate will increase by a factor of 3.
Concentration refers to the ability to focus one's attention and mental effort on a particular task or object while ignoring distractions and irrelevant information. It is an important cognitive skill that is required in a variety of tasks, including studying, problem-solving, and decision-making.
Concentration can be affected by various factors, such as the level of interest in the task, the level of fatigue, the environment, and the individual's mental and physical health. Poor concentration can lead to a range of problems, including decreased productivity, difficulty completing tasks, and impaired performance. Overall, concentration is a crucial aspect of cognitive functioning, and developing effective concentration skills can enhance performance and productivity in various aspects of life.
To learn more about Concentration visit here:
brainly.com/question/10725862
#SPJ4
What is the pOH of water?

Answers
Answer:
A. 7
(assuming the water is neutral)
What determines the maximum hardness that is obtained in a piece of steel?
Answers
The maximum hardness obtained in a piece of steel is primarily determined by its carbon content. Steel is an alloy of iron and carbon, and the carbon atoms play a crucial role in influencing the material's hardness.
When steel is heated and then rapidly cooled in a process called quenching, the carbon atoms become trapped within the iron lattice structure. This rapid cooling prevents the carbon atoms from diffusing and forming larger crystals, resulting in a harder microstructure.
The higher the carbon content in the steel, the greater the potential for hardness. Steels with higher carbon concentrations can form more carbide particles, which contribute to increased hardness.
However, it's important to note that other factors can also affect the hardness of steel, such as the presence of other alloying elements (e.g., chromium, manganese) and the specific heat treatment processes employed. These factors can influence the formation of different microstructures and phases, affecting the steel's overall hardness.
To know more about the carbon content refer here,
https://brainly.com/question/11601708#
#SPJ11
Rubbing your hands together warms them by converting work into thermal energy. If a woman rubs her hands back and forth for a total of 20 rubs, at a distance of 7.50 cm per rub, and with an average frictional force of 40.0 N, what is the temperature increase
Answers
Rubbing your hands together generates thermal energy through work conversion. In this scenario, a woman rubs her hands 20 times, each rub at a distance of 7.50 cm, and with an average frictional force of 40.0 N.
When two surfaces rub against each other, friction occurs, and work is done. In this case, as the woman rubs her hands together, the frictional force of 40.0 N is applied over a distance of 7.50 cm per rub, resulting in work being done. The work done is calculated by multiplying the force with the distance: Work = Force × Distance.
To determine the temperature increase, we need to consider the conversion of work into thermal energy. According to the first law of thermodynamics, work done on a system is converted into internal energy, which manifests as an increase in temperature. The thermal energy generated by the 20 rubs is equal to the total work done.
To calculate the temperature increase, we need to consider the specific heat capacity of the hands. Since this information is not provided, we cannot provide an exact numerical answer without making assumptions. However, it is important to note that rubbing your hands together does generate some heat, which can result in a noticeable increase in temperature.
Learn more about first law of thermodynamics here:
https://brainly.com/question/10713638
#SPJ11
NaCl molar mass? show solution pls
Answers
58.44 g/mol. One mole of sodium chloride weighs 58.44 g. (6.02 x 1023 formulas). To put it another way, it provides the grams of a substance per mole.
A molar mass A chemical compound's mass M is equal to the molecular weight (M) of the substance. MB = m/nB, where m is the total mass of a sample of a pure substance and nB is the amount of substance B expressed in moles, is the formula for this equation. Pure substance falls under the definition. You can find out how much a compound weighs in moles by looking at its molar mass. To put it another way, it provides the grams of a substance per mole.
Calculation:58.44 g/mol
The mass of a mole of sodium chloride is 6.02 x 1023 formulae.
To know more about sodium chloride visit:-
https://brainly.com/question/9811771
#SPJ1
is 8 hcl and 2 h2 products, consumers or reactants
Answers
Answer:
8 hcl and h2 is reactants.