Answers
Answer:
B. equal numbers of molecules
Explanation:
Related Questions
What is deltaG for a reaction where
DeltaG = 3.2 kJ/mol and Q = 3.3 at
295 K? (R = 8.314 J/mol K)
Answers
The free energy is 6.128 kJ/mol
What is free energy?Free energy, also known as Gibbs free energy, is a thermodynamic quantity used to describe the energy available to do work in a system
The concept of free energy is important in many areas of chemistry, including chemical thermodynamics, biochemistry, and materials science. It is used to understand and predict the behavior of chemical reactions, phase transitions, and other thermodynamic processes.
ΔG = ΔG° + RTlnQ
ΔG = 3.2 * 10^3 + (8.314 * 295 * ln(3.3)
= 3.2 * 10^3 + 2.928 * 10^3
= 6.128 * 10^3 J/mol
or 6.128 kJ/mol
Learn more about free energy:https://brainly.com/question/15319033
#SPJ1
Determine the number of moles in 4.75 X 1020 atoms of Lead
Answers
Answer:
\(\boxed {\boxed {\sf 7.89*10^{-4} \ mol \ Pb}}\)
Explanation:
To convert from moles to atoms, we must use Avogadro's Number. Avogadro's Number
6.022*10²³The number of particles (atoms, molcules, ions, etc.) in 1 mole. In this case, it is the number of atoms of lead.1. Set up ratio
We can use Avogadro's Number as a fraction or ratio.
\(\frac{6.022*10^{23} \ atoms \ Pb}{1 \ mol \ Pb}\)
2. Convert atoms to moles
Multiply the given number of atoms by the ratio.
\(4.75 *10^{20} \ atoms \ Pb *\frac{6.022*10^{23} \ atoms \ Pb}{1 \ mol \ Pb}\)
Flip the fraction so that the atoms of lead can cancel each other out.
\(4.75 *10^{20} \ atoms \ Pb *\frac{1 \ mol \ Pb}{6.022*10^{23} \ atoms \ Pb}\)
\(4.75 *10^{20} *\frac{1 \ mol \ Pb}{6.022*10^{23} }\)
\(\frac{4.75 *10^{20} \ mol \ Pb}{6.022*10^{23} }\)
\(7.88774494*10^{-4} \ mol \ Pb\)
3. Round
The original measurement of 4.75 had 3 significant figures (4, 7, and 5).
We must round our answer to 3 sig figs, which is the hundredth place for the number found.
\(7.88774494*10^{-4} \ mol \ Pb\)
The 7 in the thousandth place tells us to round the 8 up to a 9 in the hundredth place.
\(7.89*10^{-4} \ mol \ Pb\)
There are 7.89*10⁻⁴ moles of lead in 4.75*10²⁰ atoms of lead.
The molecular formula of butane is C4H10. It is obtained from petroleum and is used commonly in LPG (Liquefied Petroleum Gas) cylinders (a common source of cooking gas). It has two arrangements of carbon atoms: a straight chain and a branched chain. Using this information, draw the structure of the tertiary butyl radical that will form upon removal of a hydrogen atom. Draw the molecule on the canvas by choosing buttons from the Tools (for bonds).
Answers
Answer:
See attached picture.
Explanation:
Hello.
In this case, since butane has two common occurring structures, n-butane and isobutane, there is a way in which the tert-butyl radical can be formed upon the removal of a hydrogen from the isobutane form of butane as shown on the attached picture, wherein you can see that the radical is named by "tert" since the central carbon is bonded to three carbon atoms, that is why we classify it as tertiary. Moreover, it is a radical due to the presence of the bolded dot next to the tertiary carbon suggesting that it is very likely to bond with an other atom.
Best regards.

2. Experimental data for a simple reaction showing the rate of
change of reactant with time are given to Table 5.13.
Table 5.13 Experimental
data for a simple reaction.
Time
(min)
Concentration
(kg·m−3)
0 16.0
10 13.2
20 11.1
35 8.8
50 7.1
Show that the data gives a kinetic equation of order 1.5 and determine the rate constant.
Answers
The kinetic equation for the given reaction is first-order with respect to the reactant, and the rate constant is zero.
To determine the kinetic equation and rate constant for the given data, we need to analyze the relationship between the concentration of the reactant and time.
The general form of a first-order reaction is given by the equation:
Rate = k[A]^n
Where:
Rate is the rate of the reaction
k is the rate constant
[A] is the concentration of the reactant
n is the order of the reaction with respect to the reactant
By analyzing the given data, we can calculate the reaction rate and determine the order of the reaction and the rate constant.
Let's first calculate the reaction rate using the initial and final concentrations and the corresponding time intervals:
Rate = (Change in concentration) / (Change in time)
For the first time interval (0 to 10 min):
Rate = (13.2 kg·m^(-3) - 16.0 kg·m^(-3)) / (10 min - 0 min) = -2.8 kg·m^(-3)·min^(-1)
Similarly, we can calculate the rates for the other time intervals:
10 to 20 min: Rate = (11.1 kg·m^(-3) - 13.2 kg·m^(-3)) / (20 min - 10 min) = -2.1 kg·m^(-3)·min^(-1)
20 to 35 min: Rate = (8.8 kg·m^(-3) - 11.1 kg·m^(-3)) / (35 min - 20 min) = -2.3 kg·m^(-3)·min^(-1)
35 to 50 min: Rate = (7.1 kg·m^(-3) - 8.8 kg·m^(-3)) / (50 min - 35 min) = -1.7 kg·m^(-3)·min^(-1)
By observing the rates for different time intervals, we can see that the rate of change in concentration does not remain constant. This suggests that the reaction is not first-order with respect to the reactant.
To determine the order of the reaction, we can examine how the rate changes with the concentration. Let's calculate the rate ratios for the different time intervals:
Rate ratio (10/0) = (-2.8 kg·m^(-3)·min^(-1)) / (-2.8 kg·m^(-3)·min^(-1)) = 1
Rate ratio (20/10) = (-2.1 kg·m^(-3)·min^(-1)) / (-2.8 kg·m^(-3)·min^(-1)) ≈ 0.75
Rate ratio (35/20) = (-2.3 kg·m^(-3)·min^(-1)) / (-2.1 kg·m^(-3)·min^(-1)) ≈ 1.10
Rate ratio (50/35) = (-1.7 kg·m^(-3)·min^(-1)) / (-2.3 kg·m^(-3)·min^(-1)) ≈ 0.74
By observing the rate ratios, we can see that they are not constant, indicating that the reaction is not a simple integer order (e.g., first-order or second-order). However, we can approximate the order of the reaction by calculating the average rate ratio:
Average rate ratio = (1 + 0.75 + 1.10 + 0.74) / 4 ≈ 0.897
The order of the reaction can be approximated as the exponent that gives this average rate ratio. In this case, the order is approximately 0.897, which we can round to 1. Therefore, the kinetic equation for the reaction is:
Rate = k[A]^1.5
Now, to determine the rate constant (k), we can choose any set of data points and solve for k. Let's use the first data point at time = 0 min:
16.0 kg·m^(-3) = k * (0 min)^1.5
Since (0 min)^1.5 is zero, the right side of the equation is zero. Therefore, k must be zero as well.
For more such questions on kinetic equation visit;
https://brainly.com/question/22855016
#SPJ8
Potassium superoxide, KO2, reacts with carbon dioxide to form potassium carbonate and oxygen:
This reaction makes potassium superoxide useful in a self-contained breathing apparatus. How much O2 could be produced from 2.61 g of KO2 and 4.46 g of CO2?
Answers
First, we need to write out the balanced chemical equation for the reaction: 4 KO2 + 2 CO2 → 2 K2CO3 + 3 O2
From the equation, we can see that 4 moles of KO2 react with 2 moles of CO2 to produce 3 moles of O2. Therefore, we need to convert the given masses of KO2 and CO2 into moles:
moles of KO2 = 2.61 g / molar mass of KO2 = 2.61 g / 71.10 g/mol = 0.0367 mol
moles of CO2 = 4.46 g / molar mass of CO2 = 4.46 g / 44.01 g/mol = 0.1013 mol
Next, we need to determine the limiting reagent (the reactant that will be completely consumed in the reaction) by comparing the mole ratios of KO2 and CO2 in the balanced equation. The ratio of moles of KO2 to moles of CO2 is:
0.0367 mol KO2 / 4 mol KO2 per 2 mol CO2 = 0.0184 mol CO2
Since this ratio is less than the actual number of moles of CO2 we have (0.1013 mol), CO2 is in excess and KO2 is the limiting reagent.
Using the mole ratio from the balanced equation, we can calculate the number of moles of O2 produced:
moles of O2 = 3 mol O2 per 4 mol KO2 × 0.0367 mol KO2 = 0.0275 mol O2
Finally, we can convert the moles of O2 to grams:
mass of O2 = moles of O2 × molar mass of O2 = 0.0275 mol × 32.00 g/mol = 0.88 g
Therefore, 2.61 g of KO2 and 4.46 g of CO2 would produce 0.88 g of O2.
For more questions on: chemical
https://brainly.com/question/29886197
#SPJ11
All mass of an atom is in the what
Answers
Answer:
Protons, neutrons, and electrons
Explanation:
Both protons and neutrons have a mass of 1 amu and are found in the nucleus. However, protons have a charge of +1, and neutrons are uncharged. Electrons have a mass of approximately 0 amu, orbit the nucleus, and have a charge of -1.
What state of matter is hydrogen?
Answers
Answer:
its a gas
Explanation:
Hydrogen is the chemical element in the periodic table that has the symbol H and atomic number 1. At standard temperature and pressure it is a colorless, odorless, non-metallic, univalent, highly flammable diatomic gas. Hydrogen is the lightest and most abundant element in the universe.
In the given question, the state of matter of Hydrogen is gas in nature.
A state of matter is one of the four distinct forms in which matter can exist as solid, liquid, gas, and plasma.
Hydrogen is a chemical element that occurs naturally as a gas at standard temperature and pressure (STP). It is the lightest and most abundant element in the universe and is found in stars, gas giants, and other celestial bodies.
It is also used in various industrial processes, such as the production of ammonia and methanol, and as a fuel source in fuel cells.
Therefore, hydrogen is in the gaseous state at room temperature and normal atmospheric pressure.
Learn more about State of matter here:
https://brainly.com/question/9402776
#SPJ6
how does cohesion affect the evaporation rate of water
Answers
Answer:
Evaporation occurs because among the molecules near the surface of the liquid there are always some with enough heat energy to overcome the cohesion of their neighbors and escape. At higher temperatures the number of energetic molecules is greater, and evaporation is more rapid.
The French Le Surete included an ex-criminal, Vidocq, who put together files describing the individual modus operendi, or the _____, of each criminal.
criminal behaviors
fingerprints
home addresses
relatives who were also criminals
Answers
Answer:criminal behavior
Explanation:
Edge 2021
Answer:
Criminal Behavior
Explanation:
Which has the smaller mass, 1 mile of He atoms or 1 mole of H atoms
Answers
One mile of He atoms has a bigger mass than one mole of H atoms because there are 2.406 × 10²⁶ He atoms compared to 6.022 × 10²³ H atoms.
Whose atom count is higher 1 mole of helium or 1 mole of hydrogen?We can observe that there are roughly twice as many hydrogen atoms as helium atoms. As a result, due to the fact that hydrogen is a diatomic molecule and therefore exists in a gaseous state, there are twice as many atoms in one mole of hydrogen gas as there are in one mole of helium gas.
This can be computed using the formula:
1 mile = 1609.34 meters.
1 mile of He atoms = (4 grams/mol) x (1609.34 meters/4.0026 grams) x (6.022 × 10²³ atoms/mol) = 2.406 × 10²⁶ atoms.
1 mole of H atoms = 1 gram/mol × (atoms/mol) = atoms.
To know more about atoms visit:-
brainly.com/question/30898688
#SPJ1
The image below shows uncharged particles bouncing around.
State of Matter
Which state of matter is most likely represented in the image? (5 points)
Gas
Solid
Liquid
Plasma

Answers
This problem is providing information about the states of matter and a given diagram showing a molecular arrangement it has. Thus, we can start off by analyzing the attached file, which shows molecular arrangements and movements each state exhibits.
Solids are quite organized so that they are able to form molecular networks in which molecules vibrate but do not displace anywhere. Liquid molecules are close enough to have small movements and vibrations but are not able to form any organized network.
Gases, however, exhibit no molecular organization but large movements inside the container whose walls the gas constantly crash against. Plasma do not have any order neither yet it contains ions.
In such a way, since the given diagram do not have any apparent order or ion, we infer this is about a gas that moves into the container and crash against its walls.
Learn more:
https://brainly.com/question/18538345https://brainly.com/question/9402776
Select the structure that correspondsto the name:pentanoic acidA. CH3CH₂CH₂CH₂COOHB.C. bothCOOH

Answers
Answer
C. both
Explanation
Structures in option A and option B both corresponds to the name: pentanoic acid.
The correct answer is C. both
Correctly order the steps necessary to solve for the mass of a product, or second reactant required, given the mass of one of the reactants in a chemical process. Start with the first step at the top of the list. Place these in the proper order. O Convert the given mass into moles using molar mass. Convert moles of A to moles of B using a conversion factor derived from the balanced equation O Convert the moles of the second substance to mass using its molar mass. O Write a balanced equation for the reaction.
Answers
The correct sequence in this case would be-
Each organ system directly contributes to 1. For the reaction, write a balanced equation. Use molar mass to convert the given mass into moles.Use a conversion factor derived from the balanced equation to change moles of A into moles of B. Using the second substance's molar mass, convert the second substance's moles to mass.In terms of size and mass, molecules and atoms are incredibly small. The weight of one sample mole is known as the molar mass. The molar mass is determined by adding the atomic masses (atomic weights) of each atom in the molecule. Use the mass listed in the Periodic Table or Atomic Weight Table to determine the atomic mass for each element.To calculate the molecular mass, multiply the subscript (number of atoms) by the atomic mass of the relevant element and add the masses of all the other elements in the molecule. Typically, molar mass is expressed in either grams (g) or kilograms (kg). The amount of atoms in one mole of the 12C isotope is equal to the amount of particles in 12g (0.012 kg) of the substance. one of the most crucialTo know more about molar mass here
https://brainly.com/question/24236159
#SPJ4
The volume of a constant amount of gas at a constant temperature is increased from 3.4L to 4.0L. What is its pressure if its original was 3.0atm?
PLS HELP!! thank u
Answers
The final pressure of the gas, when its volume is increased from 3.4 L to 4.0 L at a constant temperature, is approximately 2.55 atm.
To determine the final pressure of a gas when its volume is changed while its amount and temperature remain constant, we can use Boyle's law. Boyle's law states that the pressure of a gas is inversely proportional to its volume at constant temperature.
Mathematically, Boyle's law can be expressed as:
P1 * V1 = P2 * V2
Where:
P1 = Initial pressure
V1 = Initial volume
P2 = Final pressure
V2 = Final volume
In this case, we are given:
P1 = 3.0 atm
V1 = 3.4 L
V2 = 4.0 L
We need to find P2, the final pressure.
Rearranging the formula, we have:
P2 = (P1 * V1) / V2
Substituting the values we have:
P2 = (3.0 atm * 3.4 L) / 4.0 L
P2 = 10.2 atm / 4.0 L
Calculating P2:
P2 = 2.55 atm
Therefore, the final pressure of the gas, when its volume is increased from 3.4 L to 4.0 L at a constant temperature, is approximately 2.55 atm.
Boyle's law demonstrates the inverse relationship between pressure and volume for a gas at a constant temperature. As the volume increases, the pressure decreases, and vice versa, as long as the amount and temperature of the gas remain constant.
For more such questions on pressure visit:
https://brainly.com/question/24719118
#SPJ11
How many moles of the Htion in 395 mL of 1.325 M sulfuric acid (H2SO4).
Assume the acid fully dissociates.
Answers
Answer:
1.05 mol
Explanation:
Step 1: Given data
Molarity of sulfuric acid (M): 1.325 M (1.325 mol/L)Volume of solution (V): 395 mL (0.395 L)Step 2: Calculate the moles of sulfuric acid (n)
We will use the following expression.
M = n/V
n = M × V
n = 1.325 mol/L × 0.395 L = 0.523 mol
Step 3: Calculate the moles of H⁺
H₂SO₄ dissociates completely according to the following equation.
H₂SO₄ ⇒ 2 H⁺ + SO₄²⁻
The molar ratio of H₂SO₄ to H⁺ is 1:2. The moles of H⁺ are 2/1 × 0.523 mol = 1.05 mol.
Complete and balance the molecular equation for the reaction between aqueous solutions of strontium hydroxide and lithium phosphate, and use the states of matter to show if precipitate forms.
Answers
Answer:
Explanation:
2 Li₃(PO₄) + 3Sr(OH)₂ = Sr₃(PO₄)₂ (s) + 6LiOH
precipitate of strontium phosphate is formed.
The molecular equation for the reaction between aqueous solutions of strontium hydroxide and lithium phosphate is;
2 Li₃(PO₄) + 3Sr(OH)₂(aq) = Sr₃(PO₄)₂ (s) + 6LiOHThe Strontium phosphate, Sr₃(PO₄)₂ is precipitated out of solution and exists as a solid.First, we must identify the reactants and the products formed in the chemical reaction.
According to the question:
Aqueous solutions of strontium hydroxide, Sr(OH)₂Lithium phosphate, Li₃(PO₄)The reaction between the reactants above yields; Lithium hydroxide, LiOH and Strontium phosphate, Sr₃(PO₄)₂.
The balanced chemical equation is therefore;
2 Li₃(PO₄) + 3Sr(OH)₂(aq) = Sr₃(PO₄)₂ (s) + 6LiOHThe Strontium phosphate, Sr₃(PO₄)₂ is precipitated out of solution and exists as a solid.
Read more:
https://brainly.com/question/16887565
In this list, which substance can be classified as a chemical?
Answers
Complete Question is attached below
We have that the Chemical is
Salt
From the Question we have options
Salt
Sleep
Sold
Heat
Temperature
Generally among est the options
Salt is the only chemical present as it is made of Sodium and chlorine compound
NaCl joined by an ionic bond
For more information on this visit
https://brainly.com/question/1689737?referrer=searchResults

Answer each of the following questions using the equation provided. BE SURE TO BALANCE EACH EQUATION BEFORE SOLVING ANY PROBLEMS. SHOW ALL WORK. 1. ___Cu + ___O2 ___CuO a. If 101 grams of copper is used, how many moles of copper (II) oxide will be formed? b. If 5.25 moles of copper are used, how many moles of oxygen must also be used? c. If 78.2 grams of oxygen react with copper, how many moles of copper (II) oxide will be produced?
Answers
Answer
1. 2 Cu + 1 O₂ 2 CuO
a. 1.59 moles CuO
b. 2.625 moles O₂
c. 4.88 moles CuO
Explanation
(1) The balanced equation will be
2 Cu + 1 O₂ 2 CuO
(a) If 101 grams of copper is used, how many moles of copper (II) oxide will be formed?
From the reaction;
2 moles Cu produced 2 moles CuO
Molar mass of Cu = 63.546 g/mol
Molar mass of CuO = 79.545 g/mol
That implies;
(2 mol x 63.546 g/mol) = 127.092 g Cu produced (2 mol x 79.545 g/mol) = 159.09 g CuO
Therefore, 101 g Cu will produce
\(\frac{101g\text{ }Cu}{127.092g\text{ }Cu}\times159.09g\text{ }CuO=126.43g\text{ }CuO\)Therefore the mole of CuO produced = 126.43/79.545 = 1.59 moles
(b) If 5.25 moles of copper are used, how many moles of oxygen must also be used?
From the reaction;
2 moles Cu react with 1 mole O2
Therefore, 5.25 moles Cu will react with
\(\frac{5.25\text{ }mol\text{ }Cu}{2\text{ }mol\text{ }Cu}\times1\text{ }mol\text{ }O_2=2.625\text{ }mol\text{ }O_2\)Hence, 2.625 moles of oxygen must be used.
(c) If 78.2 grams of oxygen react with copper, how many moles of copper (II) oxide will be produced?
First, convert 78.2 grams of oxygen to moles using the mole formula.
\(Mole=\frac{Mass}{Molar\text{ }mass}\)Molar mass of O₂ = 31.998 g/mol
So,
\(Mole=\frac{78.2g}{31.998g\text{/}mol}=2.44\text{ }mol\text{ }O_2\)The final step is to use the mole ratio in the balanced equation to determine the mole of CuO that will be produced.
From the equation;
1 mole of O₂ produces 2 moles of CuO
2.44 moles O2 will now produce (2.44 moles x 2) = 4.88 moles of CuO
Therefore, 4.88 moles of CuO will be produced
what structural features do cyanide and thiamine have in common that makes them capable of catalyzing the benzoin condensation
Answers
Answer:
Cyanide and thiamine do not possess specific structural features that enable them to directly catalyze the benzoin condensation reaction. However, they can participate in the catalytic process indirectly by forming complexes with other compounds or enzymes.
1. Cyanide: Cyanide ions (CN-) can act as a nucleophile, attacking the carbonyl group of aldehydes or ketones. This nucleophilic attack forms a cyanohydrin intermediate, which can undergo subsequent reactions to produce various compounds. In the benzoin condensation, cyanide can react with benzaldehyde to form a cyanohydrin, which can then undergo self-condensation to yield the benzoin product.
2. Thiamine: Thiamine, also known as vitamin B1, is not directly involved in catalyzing the benzoin condensation. However, thiamine pyrophosphate (TPP), the active form of thiamine in enzymatic reactions, can play a role. TPP is a cofactor found in enzymes called transketolases. Transketolases facilitate the transfer of two-carbon units between ketose and aldose sugars. While this is different from the benzoin condensation, the presence of TPP in the enzyme active site allows it to facilitate certain carbon-carbon bond-forming reactions.
Explanation:
It's worth noting that these examples describe the indirect involvement of cyanide and thiamine in facilitating reactions related to the benzoin condensation. Other catalysts, such as base compounds (e.g., sodium hydroxide) or other thiamine derivatives, may be used more commonly for direct catalysis of the benzoin condensation.
6.Which of the following is NOT true about the lens?

Answers
The following statement which is NOT true about the lens is:
A. It sends light to the optic nerveThe lens is a piece of glass which is used for the dispersal or refraction of light and is used to focus light onto the retina of the eye.
As a result of this, the lens is responsible for refracting of light, focusing of light and it is transparent. However, it does not send light to the optic nerve.
Therefore, the correct answer is option A
Read more here:
https://brainly.com/question/23730298
Why is a different product formed at the anode when copper sulfate is electrolysed using graphite electrodes rather than copper electrodes?
Answers
What would the approximate age of an
igneous rock that contains only 1/4 of its
original carbon-14 (half-life of carbon is
5700 years)
Answers
Explanation:
Carbon-14 has a half life of 5730 years, meaning that 5730 years after an organism dies, half of its carbon-14 atoms have decayed to nitrogen atoms. Similarly, 11460 years after an organism dies, only one quarter of its original carbon-14 atoms are still around.
to find the density of stopper I weighted it and found its mass to 4.8g. After that I filled a graduated cylinder with 32.1mL of water. After adding the stopper, the water level rose to 39.2mL. What is the density of the stopper?
Answers
Answer:
0.68g/ml
Explanation:
The density of an object is its mass per unit volume. It is calculated using the formula
Density = mass / volume
Mass of stopper weighed = 4.8g
The volume of stopper can be got by subtracting the (volume of water) from the (volume of water+stopper) i.e.
= 39.2ml - 32.1ml
= 7.1ml
Volume of stopper = 7.1ml
Density of stopper= 4.8/7.1
Density= 0.676056
Therefore, the density of the stopper is 0.68g/ml
Molecular shape and polarity can affect physical properties, such as boiling point, and they play a central role in biological function.
Answers
It is observed that the boiling point and molecular forces interact. Boiling points are a way to quantify molecular forces. Molecular forces will grow if we increase the polarization of bonds.
It is possible to raise the boiling point of a substance by increasing its molecular weight. When a liquid's vapour pressure reaches its surrounding pressure and the liquid turns into a vapour, this temperature is known as the substance's boiling point. Depending on the pressure in the environment, a liquid's boiling point can change.
An molecular forces is an attractive force that develops between the protons or positive parts of one molecule and the electrons or negative parts of another molecule. various chemical and physical characteristics.
Learn more about molecular forces here
https://brainly.com/question/24215119
#SPJ4
According to the ideal gas law, what
will be the volume of 0.25 mol of
nitrogen at 0.82 atm pressure and
57°C temperature?
Answers
Explanation:
Ideal Gas Law
PV = n R T
V = n R T / P n = moles R = gas constant = .082057 L atm / ( K mol)
T is in Kelvin
V = .25 moles * .082057 * (273.15 + 57) K / .82 atm = 8.3 moles
( two Sig Dig)
What is the formula in finding Stress?
Answers
Explanation:The unit is -
o
I
I hope it helped you.
A Brainliest please.
ASK for more
Why do we get hot when we exercise?
Answers
Identify the activated complex in the following reaction.
a. CuFeSO
b. FeFe
c. FeCuSO4
d. FeSO4
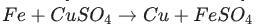
Answers
The activated complex in the following reaction is: FeCuSO4. The activated complex is a transition state that is an intermediate structure in a chemical reaction. Option C)
An activated complex is a structure that exists temporarily during a chemical reaction and corresponds to the top of the energy barrier that must be overcome for the reaction to proceed to completion.
The activated complex in the following reaction is: FeCuSO4. The activated complex is a transition state that is an intermediate structure in a chemical reaction. It is the structure with the greatest energy within the reaction process and is used to determine the rate at which the reaction occurs. An activated complex exists when the energy required to break the old bonds and form new ones has been absorbed. It has a specific configuration and energy content that is precisely defined.
A chemical reaction is the process by which atoms or groups of atoms in molecules interact to form new molecules. A chemical reaction is caused by the motion of electrons, which are negatively charged particles that surround atomic nuclei. The reaction proceeds through the formation of an intermediate species known as the transition state or activated complex. Reaction mechanisms are the sequence of steps involved in a chemical reaction. These steps describe the intermediate species formed as the reactants are converted to products. Hence option C) is correct.
for more questions on reaction
https://brainly.com/question/25769000
#SPJ8
The figure below shows a walkway with a handrail. Angle is the angle between the walkway and the horizontal, while angle is the angle between the vertical posts of the handrail and the walkway. Use the figure below to work the problem. (Assume that the vertical posts are perpendicular to the horizontal.)
Are angles and complementary or supplementary angles?
complementary
supplementary
Answers
The angles as shown are supplementary angles because the add up to 180 degrees.
What are supplementary angles?Two angles are said to be supplementary if they add up to 180 degrees. Now we know that the sum of angles on straight line is 180 degrees. If we look at the image as shown in the image attached, we can see that the angles lie on a straight line.
As such, we can conclude that the angles as shown are supplementary angles because the add up to 180 degrees.
Learn more about supplementary angles:https://brainly.com/question/13045673
#SPJ1

Find the number of neutron in an atom represented by 45X21
Answers
Answer:
My school has no stationary and my teacher has no stationary
Explanation:
cuz i am smart duh