answer this question without using numbers from the book (or anywhere else!) δs for the following reaction is negative. true or false? 2 h2(g) o2(g) → 2 h2o(g)
Answers
The statement "The following reaction is negative" is true.
Explanation :
Entropy is a measure of the disorder in a system.
Entropy also describes how much energy is not available to do work.
The more disordered a system is and the higher the entropy, the less of that system's energy is available to do work.
Although all forms of energy can be used to do work, it is not possible to use the entire amount of available energy for work.
The reaction given is:
2 H2(g) + O2(g) → 2 H2O(g)
To determine the change in entropy (δs), we follow these steps:
1. Count the moles of gas molecules on the reactant side: In this case, there are 2 moles of H2(g) and 1 mole of O2(g), giving a total of 3 moles of gas molecules.
2. Count the moles of gas molecules on the product side:
Here, there are 2 moles of H2O(g).
3. Compare the moles of gas molecules on both sides:
In this reaction, there are fewer moles of gas molecules on the product side (2 moles) than on the reactant side (3 moles).
Since there is a decrease in the number of gas molecules from reactants to products, the entropy (disorder) of the system decreases, making δs negative for this reaction.
To know more about the entropy https://brainly.com/question/13146879
#SPJ11
Related Questions
what mass of disodium tetra oxo sulphate (vi) will be dissolved in 100g of water, if the mole fraction of Na2So4 is 0.039?
Answers
Answer: 0.277g of disodium tetra oxo sulphate (vi) will be dissolved in 100g of water, if the mole fraction of Na2So4 is 0.039. ✅
PLEASEEEE GIVE BRANLIEST
Explanation:
The mass of a solute dissolved in a solvent can be calculated using the formula:
mass of solute = mole fraction of solute x mass of solvent / molar mass of solute
In this case, we are trying to find the mass of disodium tetra oxo sulphate (vi) (Na2SO4) dissolved in 100g of water, with a mole fraction of 0.039.
mass of Na2SO4 = (0.039) x (100g) / (142.04 g/mol)
mass of Na2SO4 = 0.277 g
So, 0.277 g of disodium tetra oxo sulphate (vi) will be dissolved in 100g of water, if the mole fraction of Na2So4 is 0.039. ✅
a single water molecule (h − o − h) is held together by
Answers
A single water molecule (H-O-H) is held together by covalent bonds.
In a water molecule, one oxygen atom is bonded to two hydrogen atoms. These atoms are held together by covalent bonds, which involve the sharing of electrons between the atoms. Specifically, the oxygen atom shares one electron with each of the hydrogen atoms, and each hydrogen atom shares one electron with the oxygen atom. This sharing of electrons allows each atom to have a stable electron configuration, forming a strong and stable bond.
The resulting molecule has a bent shape, with an angle of approximately 104.5 degrees between the hydrogen-oxygen-hydrogen atoms. This shape contributes to the unique properties of water, such as its polarity and hydrogen bonding capabilities.
Additionally, water molecules have a dipole moment, meaning they have a slight positive and negative charge, allowing them to interact with other polar molecules. Overall, the structure and properties of the water molecule play a crucial role in its importance for life and the environment.
To learn more about covalent bonds visit:
https://brainly.com/question/3447218
#SPJ11
a radioactive material produces 1120 decays per minute at one time, and 3.6 h later produces 140 decays per minute. what is its half-life?
Answers
The half-life of the radioactive material according to the given scenario in question is 1.2 hours.
To determine the half-life of the radioactive material, we can use the decay rate formula:
Decay rate =\(Initial\ decay\ rate * (1/2)^{(time\ elapsed / half-life)}\)
Given the initial decay rate of 1120 decays per minute and a decay rate of 140 decays per minute after 3.6 hours, we can set up the following equation:
\(140 = 1120 * (1/2)^{(3.6 / half-life)}\)
Dividing both sides of the equation by 1120, we get:
(1/8) = (1/2)^(3.6 / half-life)
To simplify further, we can rewrite (1/8) as (1/2)^3:
(1/2)^3 = (1/2)^(3.6 / half-life)
By comparing the exponents, we can deduce:
3 = 3.6 / half-life
Solving for half-life, we find:
half-life = (3.6 / 3) hours = 1.2 hours
To know more about radioactive material, here
brainly.com/question/3274297
#SPJ4
The burning of a sample of propane generated 1 04.6 kJ of heat. All of this heat was used to heat 500.0 g of water that had an initial temperature of 20.0/C. What was the final temperature of the water?
Answers
Answer: 70.0°C
Explanation:
Quantity of heat = Mass * Specific heat * Change in temperature
Quantity of heat = 104.6 KJ
Mass = 500.0 g
Specific heat of water is 4.18 J/g°C
Change in temperature assuming final temperature is x = x - 20
Units should be in grams and joules:
104,600 = 500 * 4.18 * (x - 20)
104,600 = 2,090 * (x - 20)
x - 20 = 104,600/2,090
x = 104,600/2,090 + 20
x = 69.8
= 70.0°C
What is the Hf for this reaction? 16CO2(g) + 18H2O(g) 2C8H18(l) + 25O2(g)
Answers
Answer:
10,148
Explanation:
To solve this we must be knowing each and every concept related to Enthalpy. Therefore, 10,148KJ/mol is the ΔH for formation for the reaction 16CO\(_2\)(g) + 18H\(_2\)O(g) \(\rightarrow\) 2C\(_8\)H\(_{18}\)(l) + 25O\(_2\)(g)
What is Enthalpy?Enthalpy term is basically used in thermodynamics to show the overall energy that a matter have. Mathematically, Enthalpy is directly proportional to specific heat capacity of a substances. Specific heat capacity of a substance is the amount of heat required to raise the temperature by one degree Celsius of one gram of a substance.
The balanced equation for the given chemical reaction can be given as
16CO\(_2\)(g) + 18H\(_2\)O(g) \(\rightarrow\) 2C\(_8\)H\(_{18}\)(l) + 25O\(_2\)(g)
ΔH for formation= 10,148KJ/mol
Therefore, 10,148KJ/mol is the ΔH for formation for the reaction 16CO\(_2\)(g) + 18H\(_2\)O(g) \(\rightarrow\) 2C\(_8\)H\(_{18}\)(l) + 25O\(_2\)(g)
To know more about enthalpy, here:
https://brainly.com/question/24170335
#SPJ2
What is the speed of a bird that flies 25 meters in 25 minutes?
Answers
Answer:
1mpm(1 meters per minute) Explanation: Speed:Distance/time 25/25=1 plz mark as brainliest
Answer:
1mpm(1 meters per minute)
if an atom has 35 protons in nucleus how many neutrons will it have orbiting the nueclues
Answers
Answer
Because the two particles have an equal charge, the charges will cancel out and give the atom an overall charge of 0. So, if at atom has 35 protons in the nucleus, we could expect it to have 35 electrons orbiting that nucleus.
Explanation:
explanation on redox reaction
Answers
The redox reaction must occur between an oxidizing agent and a reducing agent.
What is redox reaction?From your question, you want me to give you a general idea about redox reaction and that is what I will do. Firstly, a redox reaction is one in which one specie is oxidized and the other is reduced. The both processes have to occur simultaneously.
When we say that the both processes occur simultaneously, we mean that the electron that is lost in one process is gained in another process and all these must happen within the same system not apart from each other.
The oxidation number of one specie increases while the oxidation number of the other specie decreases. Hence, an redox reaction must occur between an oxidizing agent and a reducing agent.
Learn more about redox reaction:https://brainly.com/question/13293425
#SPJ1
Ethanol and butanol can be used as fuels for cars.
A car needs an average of 1. 95 kJ of energy to travel 1 m
Ethanol has an energy content of 1300 kilojoules per mole (kJ/mol).
Calculate the number of moles of ethanol needed by the car to travel 200 km
COULD SOMEONE EXPLAIN THIS PLEASE IM SO LOST ✋
Answers
Using dimensional analysis, the car needs 300 moles of ethanol to travel 200 km.
Dimensional analysis is the analysis of the relationship of two or more quantities of different units to calculate the unknown.
If a car needs an average of 1.95 kJ of energy to travel 1 m, and ethanol has an energy content of 1300 kilojoules per mole (kJ/mol), use dimensional analysis to calculate how many moles of ethanol are needed to travel 200 km.
Multiply all the given values in such a way that the units will be cancelled except for the unit of moles.
moles ethanol = \(200 km(\frac{1000m}{1 km})(\frac{1.95 kJ}{1 m})(\frac{mol}{1300 kJ})\)
moles ethanol = 300 mol
Learn more about dimensional analysis here: https://brainly.com/question/13078117
#SPJ4
what is the empirical formula for the ionic compound composed of calcium ions and bromide ions?
Answers
The empirical formula for an ionic compound is typically written in the form of "AxBy," where "A" is the number of atoms of the "parent" element and "x" and "y" are the numbers of atoms of the "daughter" element.
The empirical formula for an ionic compound is a simplified formula that represents the simplest ratio of the number of atoms of each element in the compound. To determine the empirical formula for an ionic compound composed of calcium ions (Ca) and bromide ions (Br), you would need to know the molar mass of each ion and the ratio of the number of moles of calcium ions to the number of moles of bromide ions in the compound.
The molar mass of calcium (Ca) is 40.08 g/mol, and the molar mass of bromide (Br) is 79.9 g/mol. Therefore, the molar mass of the compound is the sum of the molar masses of the ions:
Molar mass of compound = molar mass of Ca + molar mass of Br-
= 40.08 g/mol + 79.9 g/mol
= 119.98 g/mol
To determine the empirical formula, you would need to know the ratio of the number of moles of calcium ions to the number of moles of bromide ions in the compound. For example, if there are 2 moles of calcium ions and 1 mole of bromide ions in the compound, the empirical formula would be "Ca, Br".
Learn more about empirical formula visit: brainly.com/question/1439914
#SPJ4
the manufacture of ammonia from nitrogen and hydrogen is an exothermic reaction. which temp would give a greater yield of ammonia?
Answers
The manufacture of ammonia from nitrogen and hydrogen is an exothermic reaction, meaning that it releases heat. Therefore, the temperature at which the reaction takes place can affect the yield of ammonia.
According to the Le Chatelier's principle, if a stress is applied to a system in equilibrium, the system will adjust to counteract the stress and reach a new equilibrium state. In the case of the manufacture of ammonia, increasing the temperature would be a stress on the system.
The forward reaction of nitrogen and hydrogen combining to form ammonia is exothermic, meaning that it releases heat. Therefore, increasing the temperature will cause the equilibrium to shift towards the reverse reaction, which is endothermic, meaning that it absorbs heat. This results in a decrease in the yield of ammonia.
A lower temperature would give a greater yield of ammonia in the manufacture of ammonia from nitrogen and hydrogen. This is because the exothermic forward reaction will be favored, resulting in a higher yield of ammonia.
For more information on manufacture of ammonia kindly visit to
https://brainly.com/question/31734995
#SPJ11
MgSO (aq) + BaCl2(aq) → MgCl2(aq) + BaSO4(s)
what the role of each is in the reaction?
Answers
Answer:
oo o kwjwuwi I will send it to the future and will not have any questions please contact us if we can pm on Friday and Saturday nights in a good morning to you
Explanation:
outsiders and consideration I look forward for the future
Many plant cells have chloroplasts. Which process occurs in chloroplasts?
photosynthesis
respiration
food storage
reproduction
Answers
Answer:
photosynthesis
Explanation:
chloroplasts store sugar which is needed for photosynthesis
What does a reaction energy diagram represent ?
A. The changes in concentrations with time
B. The changes in energy during reaction reaction
C. The changes in phases with temperature
D.The changes in the reaction rate with time

Answers
Answer:
B. changes in energy during the reaction
Explanation:
y-axis measures "energy" and x-axis measures "reaction progress". Those units fit well with (B) and not so well with concentration and time (A), phases and temperature (C), or reaction rate and time (D).
Answer:
b. changes in energy during reaction
Explanation:
graph
SOMEONE HELP ME PLEASE
Enter answer for number 1
2:00 minutes
8:00 minutes

Answers
Based on factors affecting reaction rate , the time for colouring at cold temperature 5°C will be 8.00 minutes.
How does temperature affect rate of a reaction?The rate of a reaction increases with increase in temperature.
The increase in reaction rate is due to the increase in the kinetic energy of the molecules reacting.
A low temperature lowers reaction rate.
Thus, at cold temperature 5°C, the time for food colouring will be less than at room temperature.
Therefore, the time for colouring at cold temperature 5°C will be 8.00 minutes.
Learn more about reaction rate at: https://brainly.com/question/7578129
you will ask questions about the factors that have caused a rise in global
temperatures over the past century
Answers
Answer: Air pollution is the main cause of rise in temperature over the past century.
Explanation:
Due to increase in the industrialization in different countries of the world and also burning of more fossil fuels in the engines of vehicles and jets are the main reason of increasing global temperature. Both industries and vehicles produce high amount of carbondioxide which is a greenhouse gas that blocks the passage of reflected radiation from the earth surface and traps this radiation which increases the temperature of the earth.
49 grams of sulfuric acid, H2SO4, is dissolved in 1 liter of solution. Determine the molarity (M).
Answers
Answer: .5m
Explanation:
A block of metal has a mass of 51.11 g and displaces 71.46 ml of water. Calculate the density of the metal in g/ml.
Answers
Answer:
The answer is
0.07 g/mLExplanation:
The density of a substance can be found by using the formula
\(density = \frac{mass}{volume} \\ \)
From the question
mass of metal = 5.11 g
volume = 71.46 mL
The density of the metal is
\(density = \frac{5.11}{71.46} \\ = 0.071508536...\)
We have the final answer as
0.07 g/mLHope this helps you
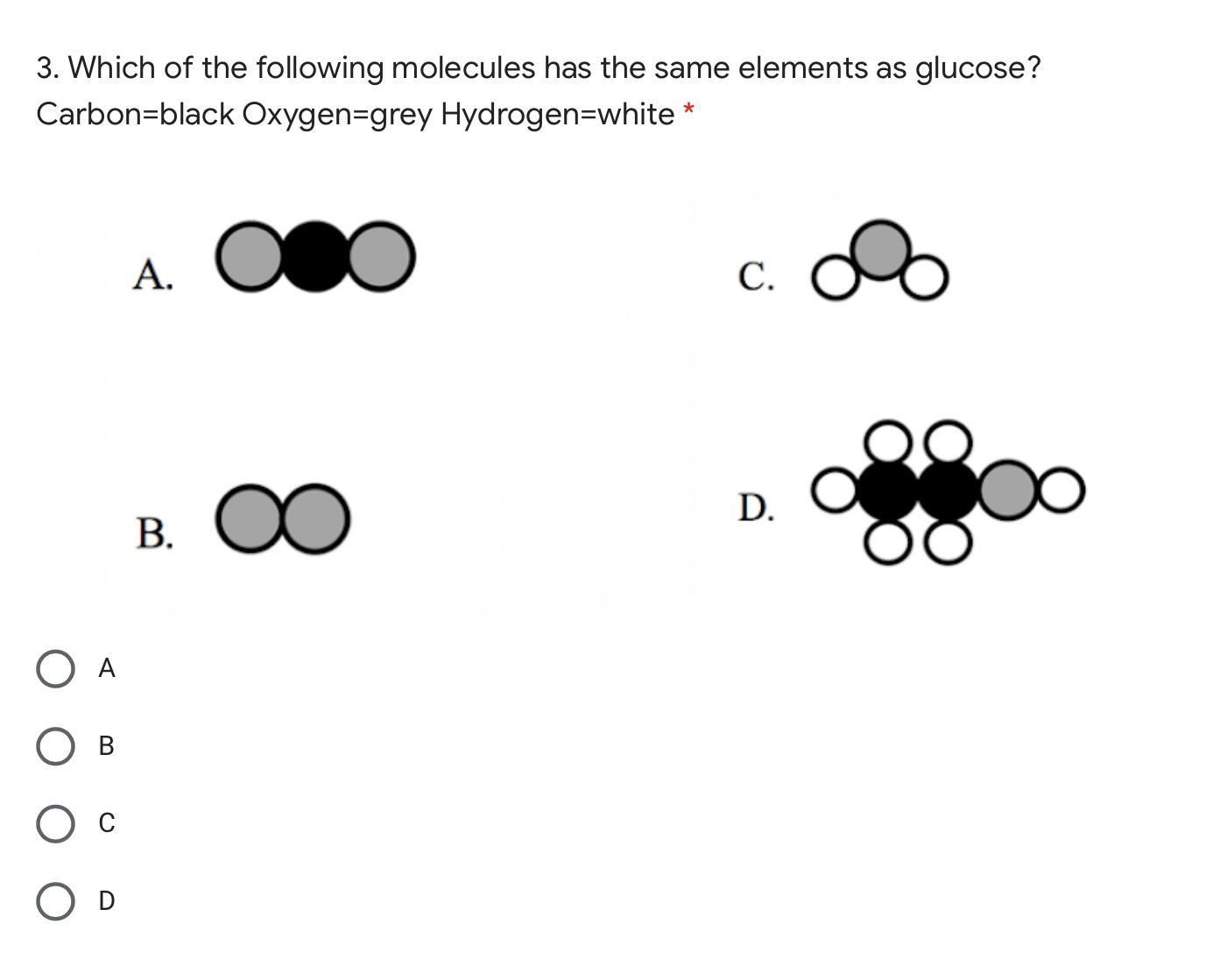
Please help me with this!
Answers
Answer:
D
Explanation:
Glucose is made of 6 Carbon atoms, 12 Hydrogen atoms, and 6 Oxygen atoms.
The equation is: C6H12O6
Answer: D
Explanation: D because it has more molecules elements then the glucose.
4. Will competition for food increase or decrease carrying
capacity?*
O A. decrease
O B. increase
Answers
hope this helps
The decrease in entropy (the DS value is negative) observed for alkene addition reactions results from:
Answers
Give a succinct description of how the various alkene reactants were combined to create the same result. The most stable intermediate carbocation will arise as a result of an electrophilic proton addition in the reaction between HCl and both alkenes.
The observed product is produced in both cases by adding Cl- to the tertiary carbocation that is produced. By figuring out the physical properties of the reactants and products, negative entropy in chemical processes may also be calculated. Gas molecules are widely spaced apart and move randomly in all directions, which results in high entropy in gases. Being a liquid converting into a solid, water freezing into ice is an entropy-reducing process. Due to the molecules' reduced freedom of movement, a solid has less chaos. A negative entropy reaction occurs when hydrogen and oxygen combine to generate water.
To learn more about carbocation please click on below link
https://brainly.com/question/13164680
#SPJ4
Round off to 3 sig figs-1638
Answers
1,640. The number is rounded up because the digit in the tenths place is 8, which is greater than or equal to 5.
What is rounded off?Rounding off is a mathematical process of replacing a number with an approximate value that has a shorter, simpler, or more explicit representation. It is often done to reduce the number of significant figures in a calculation or to make calculations easier. Rounding off is used in many everyday situations, such as calculating a restaurant bill or estimating the amount of paint needed to paint a room. It is also used in more complex calculations, such as in scientific and engineering calculations.
To learn more about rounded off
https://brainly.com/question/13993506
#SPJ1
Which of the following is the best representation of a heterogeneous mixture ?
A; sand and water
B; perfume
C; Gatorade
D; koolaid and water
Answers
Answer:
a)
Explanation:
WILL MARK BRAINLIEST WConsider two elements. Element A has a low ionization
energy of 500 kJ/mol and element B has a high ionization
energy of 1050 kJ/mol. Which element is most likely to form
a positive ion? [Select ]
In which block would you most likely find Element A?
Select)
In which block would you most likely find Element B?
[Select]
Answers
Answer:
the one with the lower ionization energy is more likely to be positive as it is easier to remove the valence electron that causes the positive charge
element a is likely in the s block as that is a low ionization energy and they get larger as you move up and to the R in the table
and element b is likely in the p block for the same reason
Explanation:
Answer the following question in the attachment please for homework that is due tomorrow

Answers
Answer:
Details on attachment.
Explanation:
See attached worksheet.

2. Which of the following is a greenhouse gas?
Answers
what of the following phases of matter has a fixed shape and volume
Answers
The condition of matter known as a solid has a distinct form and volume.
Exists a set volume for solids?A solid's particles are located in fixed spots inside a constant volume. Because the particles within a solid vibrate about predetermined positions, solids have a distinct volume and form. In a solid, the particles have strong attractor forces that prevent them from moving and hold them in place.
Any substance that is solid has a distinct form and volume. A solid's molecules are locked in place and closely spaced. Although they can still vibrate, the molecules are unable to migrate from one area of the solid to another. A solid thus cannot simply alter its volume or form.
Learn more about solid refer
https://brainly.com/question/752663
#SPJ1
Length of a year. 31,560,000.0 seconds = 3.156 X 10^7 seconds
How do I convert into scientific notation
Answers
Answer: 3.156 * 10^7
Explanation: I do not really understand your question. You answered it yourself!
Scientific notation shortens large numbers. The number right after the decimal point can only be between 1 and 9, which you did correctly. When converting to scientific notation, the exponent of ten is based on how many places you moved the decimal and the direction you moved it (left, positive; right, negative). In this case, the exponent of ten is a positive seven.
You did everything correctly :) Good job!
If you flow a solution of Na Cl- in water over an Anion resin, what will be in the outlet stream leaving the bed
Answers
If you flow a solution of Na Cl- in water over an Anion resin, what will be in the outlet stream leaving the bed High Resolution. Cost Effective Solutions. High Capacity. Efficient Purification.
What is Anion resin?A resin or polymer that serves as a medium for ion exchange is known as an ion-exchange resin or ion-exchange polymer. It is an insoluble matrix (or support structure) made from an organic polymer substrate, typically appearing as tiny (0.25-1.43 mm radius) microbeads that are white or bin color. The process is known as ion exchange because the beads are often porous, providing a wide surface area on and inside them where the trapping of ions takes place along with the concomitant release of other ions. Ion-exchange resin comes in many different varieties. Polystyrene sulfonate is the main ingredient in most commercial resins.
Many diverse separation, purification, and decontamination techniques use ion-exchange resins. The most typical examples are water filtration and water softening.
To learn more about Anion resin from the given link:
https://brainly.com/question/6521637
#SPJ4
in part 1, choose whether the following target molecule would be better made by reaction with an organolithium reagent or an organocuprate reagent. in parts 2 and 3, draw the reagents necessary to prepare this product through two different reactions.
Answers
The reaction of the ketonic molecule with alkyl lithium produces the supplied chemical more effectively. (CH3)2CHCH2Li is the organolithium reagent utilised here. The correct answer is A.
As per the question given,
Describe organolithium.
Compounds having one organic carbon–metal link are known as organometallic compounds. These chemicals belong to a class of substances that are crucial reagents in synthetic chemistry.
Compounds containing organolithium have the generic formula R-Li. Any organic chain can be R. In the alkylation of other organic molecules, they are used.
The benzyl ketone is the provided starting chemical. where the 1, 4 addition is joining the organolithium group. The hydrogen from the previous phase of hydrolysis is connected to the first carbon of the double bond, and the alkyl group is bonded to the fourth carbon.
For such more questions on Organolithium
https://brainly.com/question/21262675
#SPJ4
Note: The correct question would be as
In Part 1, choose whether the following target molecule would be better made by reaction with an organolithium reagent or an reagent. In Parts 2 and 3, draw the reagents necessary to prepare this product through two different reactions. 1st attempt Part 1 (1 point) See Periodic Table O See Hint Would this product be made more efficiently by reacting with an organolithium reagent or an organocuprate reagent? ls the reaction you chose an example of 1.2-addition or 1,4 addition? Choose one: O Organolithlum reagent...1.4-addition o Organocuprate reagent.. 1.4-addition Organolithium reagent... 1.2-addition Organocuprate reagent... 1.2-addition