4. Which of the following
is an example of a
heterogeneous mixture?
a, tea
b. oxygen
c. pizza
d. water
Answers
Answer:
water
Explanation:
Answer:
c. pizza
Explanation:
Pizza is a heterogeneous mixture because it components are not uniform.
Related Questions
How many moles are in 7.9 grams of P 2 O 5?
Answers
Answer: 0.0556555468903552 moles, you can estimate that to its nearest hundreth, or thousandth.
Explanation:
im just naturally a genius
Assume that your electrical power company gets its energy from a hydroelectric dam. Outline all of the energy changes that occurred from the falling water that turned the generators at the dam to the hot air that was produced from your hair dryer this morning.
Answers
Kinetic energy in different forms occurred from the falling water that turned the generators at the dam to the hot air that was produced from your hair dryer this morning.
What is Kinetic energy ?Kinetic energy is a form of energy that an object or a particle has by reason of its motion. If work, which transfers energy, is done on an object by applying a net force, the object speeds up and thereby gains kinetic energy.
Kinetic energy (water) to electric energy (dam) as the hydroelectric dam works.
Then, as the current travels from the plant to your crib, electric energy to heat energy (wire resistance).
Then, when you are using the dryer electric energy to heat energy (hot air) and sound energy (air particles vibrate due to the heat energy).
Therefore, Kinetic energy in different forms occurred from the falling water that turned the generators at the dam to the hot air that was produced from your hair dryer this morning.
Learn more about Kinetic energy here ;
https://brainly.com/question/12669551
#SPJ1
please answer all parts thank you
Complete the simple analysis of temperature (for which there are always observations of temperature that correspond to the contour values) in Figure 2 for the 75 and 70°F isotherms. The 80°F contour
Answers
Given Figure 2 below shows a set of contour lines for temperature, and the question wants you to complete a simple analysis of temperature. The analysis should be made for the 75 and 70°F isotherms. The 80°F contour is also to be analyzed.
Figure 2 From the image above, we can identify the following contour lines and their values:Contour line C1 is for a temperature of 60°F.Contour line C2 is for a temperature of 65°F.Contour line C3 is for a temperature of 70°F.Contour line C4 is for a temperature of 75°F.Contour line C5 is for a temperature of 80°F.Using the given information, we can then proceed to answer the questions as follows:Analysis for the 75°F isotherm Contour line C4 shows a temperature of 75°F. This means that any point lying on this contour line has a temperature value of 75°F. Therefore, we can conclude that the following regions have a temperature of 75°F:Region A: This region is enclosed by contour lines C3 and C4.
Thus, it has a temperature of 75°F.Region B: This region is enclosed by contour lines C4 and C5. Thus, it has a temperature of 75°F.Analysis for the 70°F isotherm Contour line C3 shows a temperature of 70°F. This means that any point lying on this contour line has a temperature value of 70°F. Therefore, we can conclude that the following regions have a temperature of 70°F:Region C: This region is enclosed by contour lines C2 and C3. Thus, it has a temperature of 70°F.Region D: This region is enclosed by contour lines C3 and C4. Thus, it has a temperature of 70°F.Analysis for the 80°F contourContour line C5 shows a temperature of 80°F. This means that any point lying on this contour line has a temperature value of 80°F. Therefore, we can conclude that the following regions have a temperature of 80°F:Region E: This region is enclosed by contour lines C4 and C5. Thus, it has a temperature of 80°F.
To know more about point lying visit:-
https://brainly.com/question/30174758
#SPJ11
carbon-carbon covalent bonds, such as the ones in carbohydrates and lipids, are _____ and have _____.
Answers
Answer:
Weak; a lot of potential energy.
Explanation:
Carbon-carbon covalent bonds, such as the ones in carbohydrates and lipids, are weak and have potential energy.
What is covalent bond?In chemistry, a covalent bond is a chemical connection between two ions or atoms in which their respective electron pairs are shared. A molecular bond is another name for a covalent link.
Several other chemical species, including radicals and macromolecules, may also include this kind of connection. Irving Langmuir invented the term "covalence" around 1919 to characterize the quantity of electron pairs exchanged by nearby atoms, but the phrase "covalent bond" wasn't used until 1939. Carbon-carbon covalent bonds, such as the ones in carbohydrates and lipids, are weak and have potential energy.
Therefore, carbon-carbon covalent bonds, such as the ones in carbohydrates and lipids, are weak and have potential energy.
To learn more about covalent bond, here:
https://brainly.com/question/10777799
#SPJ2
When mixing 5.0 moles of HZ acid with water up to complete a volume of 10.0 L, it is found that at
reach equilibrium, 8.7% of the acid has become hydronium. Calculate Ka for HZ. (Note: Do not assume is disposable. )a. 1.7×10^−3
b. 9.5×10^−2
C. 2.0×10^−2
d. 4.1×10^−3
e. 3.8×10^−3
f. 5.0×10^−1
Answers
therefore the correct option is d) 4.1×10⁻³.
Given that the initial concentration of HZ is 5.0 moles and at equilibrium, 8.7% of the acid has become hydronium.
The concentration of HZ that has not reacted is (100% - 8.7%) = 91.3%.
The final concentration of HZ is 5.0 × 0.913 = 4.565 moles.
The final concentration of the hydronium ion is 5.0 × 0.087 = 0.435 M.HZ ⇌ H+ + Z-Ka
= [H+][Z]/[HZ]Ka
= [H+][Z]/[HZ]
= [0.435]² / 4.565
= 0.041
Which is the same as 4.1 × 10-3.
We know that HZ is an acid that will partially ionize in water to give H+ and Z-.
The chemical equation for this reaction can be written as HZ ⇌ H+ + Z-.
The acid dissociation constant (Ka) of HZ is the equilibrium constant for the reaction in which HZ ionizes to form H+ and Z-.Thus, Ka = [H+][Z]/[HZ].
The given problem is a typical example of the dissociation of a weak acid in water. We are given the initial concentration of HZ and the concentration of hydronium ions at equilibrium.
To find the equilibrium concentration of HZ, we can use the fact that the total amount of acid is conserved.
At equilibrium, 8.7% of HZ has dissociated to give hydronium ions.
This means that 91.3% of the original HZ remains unreacted.
Therefore, the concentration of HZ at equilibrium is 5.0 × 0.913 = 4.565 M.
The concentration of hydronium ions at equilibrium is 5.0 × 0.087 = 0.435 M.
Using the equation Ka = [H+][Z]/[HZ], we can substitute the values of the concentrations and the equilibrium constant into the equation and solve for Ka.
Ka = [H+][Z]/[HZ]
= [0.435]² / 4.565
= 0.041 or 4.1 × 10-3.
The answer is d) 4.1 × 10-3.
To know more about hydronium visit:
https://brainly.com/question/14619642
#SPJ11
My mother packed my woollen clothes in the summer season. She added a few white
coloured balls along with my clothes in the almirah . In the winter season when I opened the
almirah most of the balls had disappeared and a very few were left that had considerably
decreased in size. I was amazed.
a. What was the composition of those balls?
b. Why had those balls disappeared/decreased in size?
c. What was the purpose of putting those balls along with clothes?
Answers
a. The balls were most likely mothballs, which are commonly made of naphthalene or paradichlorobenzene.
b. The balls disappeared/decreased in size due to sublimation, where they transitioned from solid to gas without becoming a liquid.
c. The purpose of putting the balls along with clothes was to repel moths and other insects, protecting the woolen clothes from damage during storage.
a. The composition of the balls was most likely mothballs, which are typically made of a chemical compound called naphthalene or paradichlorobenzene.
b. The balls disappeared or decreased in size due to sublimation. Mothballs undergo a process called sublimation, where they transition from a solid to a gas state without going through the liquid phase. This means that over time, the mothballs slowly vaporize and dissipate into the air, resulting in their decrease in size or disappearance.
c. The purpose of putting those balls along with clothes was to prevent damage from moths and other insects. Mothballs contain chemicals that release a strong odor, which is unpleasant to insects. The odor acts as a deterrent and helps protect the clothes from being damaged by moth larvae or other pests that feed on natural fibers like wool. By placing the mothballs in the storage area, it creates an environment that repels these insects and helps preserve the quality of the woollen clothes during storage.
Know more about paradichlorobenzene here:
https://brainly.com/question/14274494
#SPJ8
How do you increase the absorbance of a sample with a fixed concentration?.
Answers
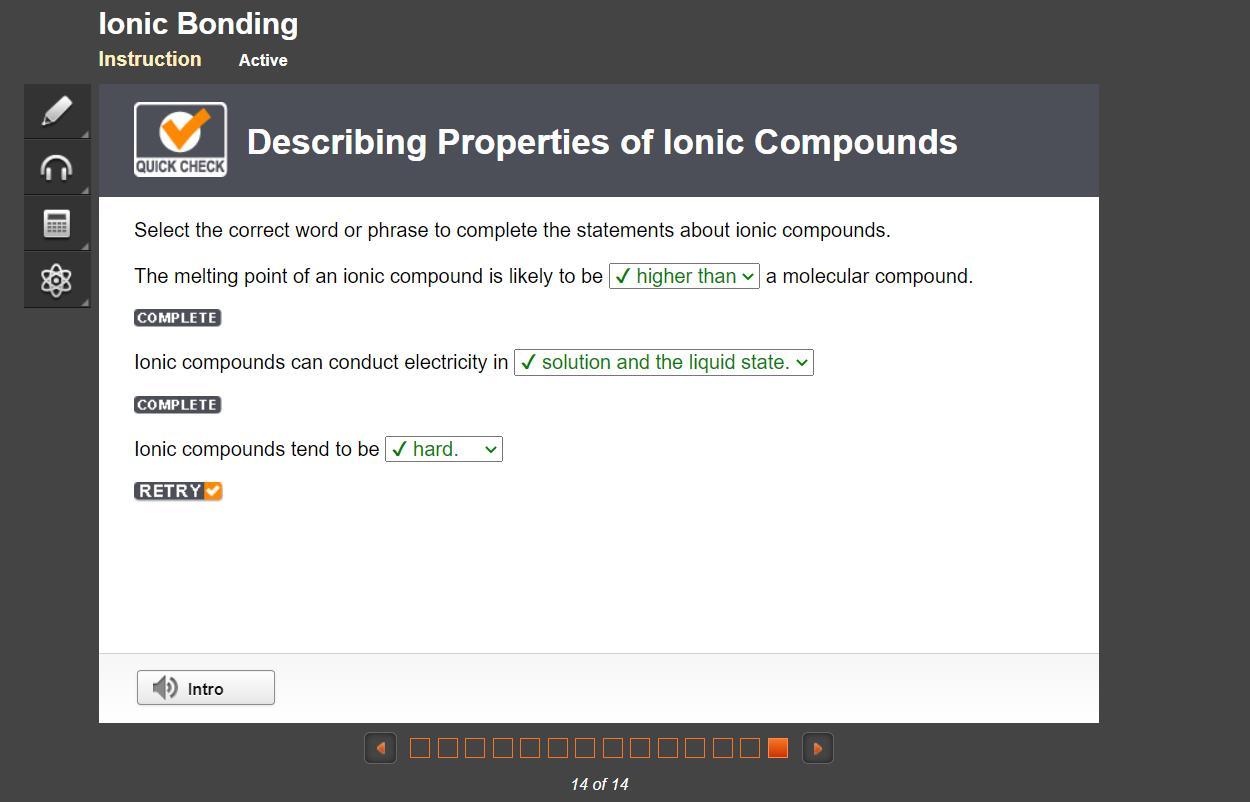
The melting point of an ionic compound is likely to be (answer) a molecular compound.
options:
higher than
lower than
the same as
Answers
Wish me luck
Answer:
Higher than
solution and the liquid state
hard
Explanation:
Using the reaction below, how many molecules of lithium nitrate (LiNo3) will be needed to react with 2.35 grams of lead (IV) sulfate, Pb (SO4)2?equation in photo

Answers
From the reaction, each Pb(SO₄)₂ reacts with 4 LiNO₃.
So, first, we need to figure ot how many moles of Pb(SO₄)₂ we have in 2.35 grams.
For this, we need to use the molar masses of its elements to find its molar mass:
\(\begin{gathered} M_{Pb\mleft(SO_4\mright)_2}=1\cdot M_{Pb}+2\cdot(1\cdot M_S+4\cdot M_O)=(1\cdot207.2+2\cdot(1\cdot32.065+4\cdot15.9994))g\/mol \\ M_{Pb(SO_4)_2}=399.3252g\/mol \end{gathered}\)Using it, we can calcualte the number of moles of Pb(SO₄)₂:
\(\begin{gathered} M_{Pb\mleft(SO_4\mright)_2}=\frac{m_{Pb\mleft(SO_4\mright)_2}}{n_{Pb\mleft(SO_{4}\mright)_{2}}} \\ n_{Pb\mleft(SO_4\mright)_2}=\frac{m_{Pb\mleft(SO_4\mright)_2}}{M_{Pb\mleft(SO_{4}\mright)_{2}}}=\frac{2.35g}{399.3252g\/mol}=0.005884\ldots mol \end{gathered}\)Using the stoichiometry, we have:
LiNO₃ --- Pb(SO₄)₂
4 --- 1
\(\begin{gathered} \frac{n_{LiNO_3}}{4}=\frac{n_{Pb\mleft(SO_4\mright)_2}}{1} \\ n_{LiNO_3}=4\cdot n_{Pb\mleft(SO_4\mright)_2}=4\cdot0.005884\ldots mol=0.023539\ldots mol \end{gathered}\)Now that we know the number of moles of LiNO₃, we can calculate how many LiNO₃ are needed by using the Avogadro's Number:
\(N_{LiNO_3}=N_A\cdot n_{LiNO_3}\)Avogadro's Number is approximately 6.022 x 10²³ mol⁻¹, so, using this value, we have:
\(N_{LiNO_3}=6.022\times10^{23}mol^{-1}\cdot0.023539\ldots mol=1.41756\ldots\times10^{22}\approx1.42\times10^{22}\)So, we need approximately 1.42 x 10²² LiNO₃.
What is the percent oxygen in something made of 2 mole of copper and 1 mole of oxygen called copper (l) oxide?
Answers
Answer:
20.114%
Explanation:
Percent composition by element Element Symbol Mass Percent Copper Cu 79.886% Oxygen O 20.114%
What happens to the density of a substance when it is heated? Explain your answer.
Answers
Heating a substance causes molecules to speed up and spread slightly further apart, occupying a larger volume that results in a decrease in density. Cooling a substance causes molecules to slow down and get slightly closer together, occupying a smaller volume that results in an increase in density.
From: www.middleschoolchemistry.com
How do you know something is there if you can't see it?
Answers
Answer:
Explanation:
Use your other senses such as touch or smell, does it react in different temperatures, is it then visible under a microscope, does it react with different elements or gases? Think outside the box.
Answer:
we know that someone or something is near us even when we cant see it you see our self conscious minds can pick up on the slightest detail even when we dont pick up on it also you can thank the six senses as well for why we can tell something or someone is there
Explanation: i watch criminal minds and pay attention to the random facts spencer reid spits out
Could someone explain this for me I don't understand?

Answers
Answer:
Ok so I assume they gave u a reaction before asking this question. Look at that reaction and try to figure out what substances are changing in valency, if there is a substance(s) that doesn't change its valency then it's a spectator ion.
Which of the following properties is a common property of acids?
a) pH values < 7
b) insoluble in water
c) does not conduct electricity
d) does not react with metals
Answers
The correct answer is a) pH values < 7. Acids are substances that donate hydrogen ions (H+) when dissolved in water, which increases the concentration of H+ ions in the solution.
This excess of H+ ions causes the pH of the solution to decrease below 7, which is considered neutral. Therefore, solutions with pH values below 7 are acidic, while solutions with pH values above 7 are basic or alkaline.
Insolubility, lack of conductivity, and lack of reactivity with metals are not properties common to all acids. Some acids can be soluble in water, conduct electricity, or react with metals, depending on their specific chemical properties.
To know more about pH values, visit :
https://brainly.com/question/28580519
#SPJ1
What pathway does the air that enters your body take?
a. nose, esophagus, larynx, bronchi, lungs
b. nose, pharynx, bronchi, larynx, trachea
c. nose, pharynx, lungs, trachea, larynx
d. nose, pharynx, larynx, trachea, bronchi, lungs
Answers
Answer:
D
Explanation:
The air that we breathe in enters the nose or mouth, flows through the throat (pharynx) and voice box (larynx), and enters the windpipe (trachea). The trachea divides into two hollow tubes called bronchi.
Organisms use their sensory organs to learn about their environment. how might an organism use their senses to avoid danger
Answers
Organisms use their senses to avoid danger by visually detecting threats, listening for warning signals, smelling potential hazards, feeling physical contact, tasting toxins, and even electroreception in some aquatic species.
An organism can use its senses to avoid danger in various ways:
Sight: The organism may visually detect predators or hazardous objects and respond by altering its path or taking evasive actions.
Hearing: By listening for specific sounds, such as predator calls or warning signals, the organism can detect danger and react accordingly.
Smell: The organism may detect odors associated with potential threats, such as the scent of a predator or a toxic substance, and use this information to avoid danger.
Touch: Sensing physical contact or changes in the environment, the organism can respond by moving away from potential harm or by using reflexes to protect itself.
Taste: Some organisms can taste or chemically detect harmful substances, allowing them to avoid ingesting toxins or spoiled food.
Electroreception: Certain aquatic organisms, like sharks or electric fish, can sense electric fields generated by other animals, helping them detect potential threats or prey.
It's important to note that the specific senses and mechanisms used by organisms to avoid danger can vary greatly depending on their species and ecological niche.
To know more about aquatic species refer here
https://brainly.com/question/8603354#
#SPJ11
Consider a biochemical reaction that is taking place in a 0. 1 M buffer. The initial pH is 7. 4, and the pKa of the buffer is 7. 2. If, in a final reaction volume of 1. 0 mL, 10 micromol of protons are generated, what would be the final pH of the solution?
Answers
The final pH of the solution would be 2.2
The final pH of the solution can be calculated using the Henderson-Hasselbalch equation, which states that the pH of a buffer solution is equal to the pKa of the buffer plus the logarithm (base 10) of the ratio of the concentration of the buffer's conjugate base to the concentration of its acid.
In this case,
The buffer is 0.1 M The pKa of the buffer is 7.2.The initial pH of the solution is 7.4, which means that the buffer is in its protonated form (the acid) at the start of the reaction.
When 10 micro mols of protons are generated, the buffer will lose protons and become the conjugate base. Therefore, the concentration of the conjugate base will increase and the concentration of the acid will decrease.
To calculate the final pH, we use the Henderson-Hasselbalch equation:
pH = pKa + log([conjugate base]/[acid])
We know that the pKa = 7.2, so the final pH will be equal to 7.2 + log([conjugate base]/0.1 - [conjugate base]).
To find the [conjugate base], we can use the stoichiometry of the reaction. We know that the reaction generates 10 micro mols of protons. We also know that the buffer has a 1:1 stoichiometry (1 acid: 1 conjugate base)
So [conjugate base] = 1010^-6 mol / 1 = 1010^-6 M
Therefore, the final pH = 7.2 + log(10*10^-6/0.1)
= 7.2 + log(10^-5) = 7.2 - 5 = 2.2
So the final pH of the solution would be 2.2
Learn more about the Henderson-Hasselbalch equation here:
https://brainly.com/question/13423434
#SPJ4
4. Ava is an amazing swimmer and can swim 1000 meters in 360 seconds. What is her average speed in m/s?
Answers
Answer:
Speed =Distance /Time =1000/360=2.78m/s
Make a claim about why scientists might have two ways for thinking about Earth's layers. Summarize evidence to support the claim and explain your reasoning.
Answers
Answer:
Direct evidence from rock samples and indirect evidence from seismic waves may account for why scientists have two ways of thinking about earth's layers
Explanation:
Scientists who study about the earth's structure and components called geologists have two main types of evidence to learn about Earth's interior namely
1. direct evidence from rock samples
2. indirect evidence from seismic waves
Direct evidence From Rock Samples
Scientists examine rocks from inside the Earth and these rocks give them clues about Earth’s structure. Some scientists have drilled holes as much as 12 kilometers into Earth and bring up samples of rock. From these samples, they can make inferences about conditions deep inside Earth, where these rocks formed. In addition, forces inside Earth sometimes blast rock to the surface from depths of more than 100 kilometers. These rocks from deep within the Earth provide more information about the interior.
Indirect evidence From Seismic Waves
Since scientist cannot look inside Earth, they have devised an indirect methods of observation by using seismic waves. When earthquakes occur, they produce seismic waves. Scientists record the seismic waves and study how they travel through Earth. The speed of seismic waves and the paths they take reveal the structure of the planet. Using data from seismic waves, scientists have learned that Earth’s interior is made up of several layers with each layer surrounding the layers beneath it.
How many molecules are in 1 mole of nitrogen sulfide
Answers
Answer:
46.07
Explanation:
im not 100 sure this is right almost tho
please mark brainliest
What would be the bond angle if the molecular geometry were bent and had only one lone pair of electrons
Answers
120°
Bent molecular geometry
Examples H2O, SO2
Point group C2v
Coordination number 2
Bond angle(s) 90°<θ<120°
What is the percentage by mass of copper in copper oxide (CuO)?
Answers
Answer:
79.89%
Explanation:
Copper has a molar mass of 63.55
Oxygen has a molar mass of 16.00
If you add these you get 79.55
To get the mass percent of copper divide copper by the whole. 63.55/79.55*100= 79.89%
Based on Reference Table F, which salt is the strongest electrolyte?
1.
CaCO3
2.
Na2SO4
3.
AgCI
4.
Zn3(PO4)2
Answers
Answer:
Na2SO4
Explanation:
Na2SO4 dissociates completely in solution to yield ions therefore it is the strongest electrolyte.
The question is incomplete as the table is missing but I will try to explain as generally as possible. Recall that an electrolyte is a compound that conducts electricity in solution.
Also recall that the number of ions available in solution determines the electrolytic strength of a substance. Hence, Na2SO4 dissociates completely in solution to yield ions therefore it is the strongest electrolyte.
Learn more about electrolytes: https://brainly.com/question/1301963
7.) The temperature of a hot cup of coffee in degrees Fahrenheit is modeled by the function T(t) = 70+ 142ekt, where t is time measured in minutes and T(t) is the temperature (°F). The coffee temperature at 10 minutes was 110° F.
a) Solve for the k value
b) What is the T(t) at 19.5 minutes?
8) Lidocaine is commonly used by dentists to numb a patient's inner cheek or gum. Suppose a person goes to the dentist and receives a dosage of 200 mg and that the half-life of Lidocaine is about 1.5 hours.
a) Solve for k in L(t) = aekt.
b) Create the exponential model L(t) = aekt
c) Using your exponential model from part b, how long will it take for the amount of Lidocaine to reduce to 20 mg? Round final answer to the tenths
Answers
a) To solve for the k value in the equation T(t) = 70 + 142ekt, we can use the given information that the coffee temperature at 10 minutes was 110°F.
Substituting t = 10 and T(t) = 110 into the equation, we have:110 = 70 + 142ek(10). Subtracting 70 from both sides, we get: 40 = 142ek(10). Dividing both sides by 142, we have: ek(10) = 40/142. Taking the natural logarithm (ln) of both sides, we get: ln(ek(10)) = ln(40/142). Simplifying, we have: k(10) = ln(40/142). Dividing both sides by 10, we get: k = ln(40/142) / 10. Using a calculator, we find that k ≈ -0.0131. b) To find T(t) at 19.5 minutes, we can substitute t = 19.5 into the equation T(t) = 70 + 142ekt: T(19.5) = 70 + 142e(-0.0131)(19.5) Using a calculator, we can evaluate the expression to find T(19.5) ≈ 99.6°F. a) The decay of Lidocaine can be modeled using the equation L(t) = aekt. Given that the half-life of Lidocaine is about 1.5 hours, we can use this information to solve for the k value. Using the half-life formula, we know that: t1/2 = (ln 2) / k. Substituting t1/2 = 1.5 hours, we have: 1.5 = (ln 2) / k. Solving for k, we get: k = (ln 2) / 1.5. Using a calculator, we find that k ≈ 0.4621. b) The exponential model for Lidocaine decay is given by : L(t) = aekt. c) To find how long it will take for the amount of Lidocaine to reduce to 20 mg, we can substitute L(t) = 20 and solve for t. 20 = 200e0.4621t. Dividing both sides by 200, we have: 0.1 = e0.4621t. Taking the natural logarithm (ln) of both sides, we get: ln(0.1) = 0.4621t. Simplifying, we have: t = ln(0.1) / 0.4621. Using a calculator, we find that t ≈ 2.7 hours. Rounded to the tenths, it will take approximately 2.7 hours for the amount of Lidocaine to reduce to 20 mg.
To learn more about temperature, https://brainly.com/question/16999043
#SPJ11
NaHCO3+HCI--->NaCI+H2O+CO2
Percent yield:93.4%
how would the percent yield be affected if some sodium hydrogen carbonate is left unreacted? explain
Answers
Answer:
Explanation:
percent yield is ratio of actual yield or experimental yield divided by theoretical yield multiplied by 100 .
percent yield of 93.4 % means , the actual yield is 93.4 % what was expected from the reaction on the basis of given chemical reaction .
If in the experimental process , some sodium hydrogen carbonate is left unreacted due to absence of reactant HCl which is also required to obtain product , the percent yield will be increased if the required HCl is also provided .
Hence the percent yield will be increased if required HCl is made available .
Any 1 help with Chemistry here??
Answers
When handling ready to eat food, the best alternative to using latex-free gloves is:
Answers
The best alternative to using latex-free gloves when handling ready-to-eat food is using nitrile gloves.
Nitrile gloves are a suitable alternative to latex gloves for handling ready-to-eat food. Nitrile is a synthetic material that offers similar benefits to latex in terms of flexibility and dexterity. Nitrile gloves are also resistant to punctures and chemicals, providing a protective barrier against potential contamination. Moreover, nitrile gloves are considered hypoallergenic and do not cause the same allergic reactions as latex gloves, making them safe for individuals with latex allergies or sensitivities.
When handling ready-to-eat food, it is crucial to maintain hygiene and prevent cross-contamination. Wearing gloves is an important practice to minimize the risk of transmitting harmful microorganisms. By choosing nitrile gloves as an alternative to latex gloves, one ensures the safety of individuals who may be allergic to latex while maintaining the necessary level of protection when handling food. Nitrile gloves are widely available and commonly used in food handling settings, making them a suitable and reliable choice for ensuring food safety and hygiene.
Learn more about Nitrile gloves here: brainly.com/question/31718871
#SPJ11
Procathepsin B is a lysosomal protease that is first translated as a proenzyme. On autocleavage it is fully activated. Procathepsin B is a. a zymogen b. in the T state after autocleavage Ocan allosteric enzyme Od. Inactive at low pH Trypsin, chymotrypsin, and elastase are all serine proteases that cleave after different amino acids What is responsible for the substrate specificity? a. The substrate binding pockets accommodate different amino acids. b. Different amino acids involved in the catalytic trad c. Different catalytic mechanisms d. Each protease is made in a different cell type Which amino acid is NOT a target for kinases? a. Ser b. Asp O c Thr O d. Tyr QUESTION 7 Which of the following is best described as a prosthetic group? a. Zn2+ b. H
Answers
1. Procathepsin B is a lysosomal protease that is first translated as a proenzyme. On autocleavage, it is fully activated. Procathepsin B is a. a zymogen b. in the T state after autocleavage Ocan allosteric enzyme Od. Inactive at low pH
Answer: Procathepsin B is a zymogen.
Explanation: A zymogen is an inactive enzyme precursor that becomes active upon a biochemical modification such as autocleavage.
2. Trypsin, chymotrypsin, and elastase are all serine proteases that cleave after different amino acids. What is responsible for substrate specificity?
Answer: The substrate binding pockets accommodate different amino acids.
Explanation: Each protease has a unique substrate binding pocket that specifically recognizes and binds to certain amino acids, leading to substrate specificity.
3. Which amino acid is NOT a target for kinases?
Answer: Asp.
Explanation: Kinases primarily target the amino acids serine (Ser), threonine (Thr), and tyrosine (Tyr) for phosphorylation. Aspartic acid (Asp) is not a typical target for kinases.
4. Which of the following is best described as a prosthetic group?
Answer: Zn2+.
Explanation: A prosthetic group is a non-polypeptide unit tightly and permanently attached to a protein and required for its biological activity. Zn2+ is an example of a prosthetic group, as it is a metal ion that plays a crucial role in the function of many enzymes.
to know more about proteases refer here:
https://brainly.com/question/19053549#
#SPJ11
The initial pressure of a balloon floating in the air is 0.53 atm. After the balloon has reached a certain point in the sky, the volume of the air particles in the balloon is 4.3 liters at a final pressure of 0.42 atm. What was the initial volume of the balloon?
Answers
Answer:
The initial volume of the balloon is 3.41 L.
Explanation:
The gas laws are a set of chemical and physical laws that allow determining the behavior of gases in a closed system. The parameters evaluated in these laws are pressure, volume, temperature, and moles.
As the volume increases, the gas particles (atoms or molecules) take longer to reach the walls of the container and therefore collide with them fewer times per unit of time. This means that the pressure will be lower because it represents the frequency of collisions of the gas against the walls. In this way pressure and volume are related, determining Boyle's law which says:
"The volume occupied by a certain gaseous mass at constant temperature is inversely proportional to pressure"
Boyle's law is expressed mathematically as:
Pressure * Volume = constant
or P * V = k
Now it is possible to assume that you have a certain volume of gas V1 that is at a pressure P1 at the beginning of the experiment. If you vary the volume of gas to a new value V2, then the pressure will change to P2, and it will be fulfilled:
P1 * V1 = P2 * V2
In this case:
P1= 0.53 atmV1= ?P2= 0.42 atmV2= 4.3 LReplacing:
0.53 atm* V1= 0.42 atm* 4.3 L
Solving:
\(V1=\frac{0.42 atm* 4.3 L}{0.53 atm}\)
V1= 3.41 L
The initial volume of the balloon is 3.41 L.
A chemist samples a river's water to measure the amount of fertilizer runoff from the area farms. In a 0.0790 L sample, the chemist measures 389μg of nitrate. Express the concentration of nitrate in parts per million ( ppm ) and parts per billion (ppb). Assume the density of the river water sample is 1.00 g/mL. concentration: concentration:
Answers
Therefore, the concentration of nitrate in the given river's water sample is 4.92 ppm and 4.92 ppb.
Given data:
Volume of the sample taken, V = 0.0790 L
Density of the river water sample, d = 1.00 g/mL
Concentration of nitrate, C = ?
Mass of nitrate, m = 389 μg or 0.389 mg (1 μg = 10⁻⁶ g)
1 part per million (ppm) = 1 mg/L
1 part per billion (ppb) = 1 μg/L
Concentration (C) is defined as the ratio of the mass of solute to the volume of the solution.
Hence, the formula for concentration is:
C = m/V
Substituting the given values, we get:
C = 0.389 mg / 0.0790 L
C = 4.92 mg/L
To express the concentration of nitrate in parts per million (ppm) and parts per billion (ppb), we need to convert the given concentration in mg/L to ppm and ppb.
Parts per million (ppm):1 ppm = 1 mg/L
Hence, the concentration in ppm is:
C = 4.92 mg/L = 4.92 ppm
Parts per billion (ppb):1 ppb = 1 μg/L
Hence, the concentration in ppb is:
C = 389 μg / 0.0790 L
C = 4.92 μg/L
C = 4.92 ppb
to know more about density visit:
https://brainly.com/question/29775886
#SPJ11