2.3.12mL =
L*
O
a. .312
O b.31.2
O
C..00312
O d..0312
Answers
Answer:
0.00312 L
Explanation:
1 liter = 1,000 ml
3.12 ml*(1 liter/1,000ml) = 0.00312 L
C
Related Questions
When sugar is fermented, alcohol is produced. What other substance is produced? Also.. What effects does this substance have in (i) baking (ii) beer brewing.
Answers
ANSWER: It's (ii) I do believe
The sugar is fermented and the ethanol and carbon dioxide are produced. The fermentation of ethanol is used in bread baking and the fermentation found in grapes makes beer.
What is fermentation?Fermentation is the mechanism by which sugar is converted into alcohol. Ethanol Fermentation can be described as a biological process that converts sugars such as glucose, sucrose, and fructose into cellular energy by producing ethanol and carbon dioxide as a side effect.
Alcoholic fermentation is called anaerobic since yeast conducts this transformation in the absence of oxygen. The fermentation of ethanol has applications such as the manufacture of alcoholic drinks, ethanol fuel manufacture, and bread baking.
Fermentation of the natural sugars can be seen in grapes creating wine or beer as well as in apples and pears producing cider and Perry, respectively. Alcoholic fermentation takes place in species of fish, where it offers energy when oxygen is scarce.
Learn more about fermentation, here:
https://brainly.com/question/13050729
#SPJ6
explain how your data illustrates the idea that the half-life for decayr of a given isotope is constant
Answers
The half-life for decay of a given isotope is constant because the number of counts decreases by half after a certain amount of time.
Half -life of a substance is defined as the time which is required for half of the quantity of a radioactive isotope to get decayed.It is a term which is used in nuclear chemistry for describing how quickly unstable atoms undergo radioactive decay into other nuclear species by emitting particles or the time which is required for number of disintegrations per second of radioactive material to decrease by one half of its initial value.
Learn more about half life,here:
https://brainly.com/question/24710827
#SPJ4
At 500.0 K, one mole of gaseous ONCl is placed in a one-liter container. At equilibrium, it is found that 5.3% of the ONCl had split into NO and Cl2, according to the equation shown here:
2ONCl (g) <=> 2NO (g) + Cl2 (g)
What is the equilibrium constant?
a) 5.6e-2
b) 9.5e-1
c) 1.2e4
d) 8.3e-5
e) 1.6e-3
Answers
The equilibrium constant for the given reaction is 1.2e4 (option c).
The equilibrium constant (K) is a value that represents the ratio of product concentrations to reactant concentrations at equilibrium. In this case, the equilibrium constant is determined by the expression [NO]^2 * [Cl2] / [ONCl]^2, where [NO], [Cl2], and [ONCl] represent the concentrations of the respective species.
Given that 5.3% of the ONCl has split into NO and Cl2 at equilibrium, we can assume that the concentration of ONCl at equilibrium is reduced by 5.3% (or 0.053) and the concentrations of NO and Cl2 are increased by the same amount. Therefore, at equilibrium, the concentrations of NO and Cl2 are 0.053 and the concentration of ONCl is (1 - 0.053).
Plugging these values into the equilibrium constant expression, we get (0.053)^2 * (0.053) / (1 - 0.053)^2, which simplifies to approximately 1.2e4.
Hence, the equilibrium constant for the given reaction is 1.2e4 (option c).
learn more about equilibrium constant here; brainly.com/question/29809185
#SPJ11
The volume of a candy bar is 55cm^3. The mass of the candy bar is 70g. What is the density of the candy bar in g/cm^3
Answers
Answer:
1.3 g/cm3
Explanation:
What does it mean if elements are in the same period.
Answers
If elements are in the same period, it implies that they have the same number of atomic orbitals. It further indicates that these elements have their valence electrons in the same outermost electron shell.
Elements in a particular period have the same number of atomic orbitals. The number of orbitals increases from left to right across the period. Thus, the first period has one orbital, the second has two orbitals, and so on.Each orbital can hold up to two electrons, thus the number of electrons in an element increases from left to right across a period. When electrons are added to the same shell, they experience increased effective nuclear charge because of the increasing number of protons.
Consequently, the atomic size becomes smaller from left to right across a period. The properties of elements within a period are mainly determined by their valence electrons, which are found in the same outermost electron shell. As the number of valence electrons increases from left to right, elements become less metallic and more non-metallic. This occurs because metals tend to lose electrons, while non-metals tend to gain electrons to attain the noble gas electronic configuration.
To know more about atomic visit:
https://brainly.com/question/1566330
#SPJ11
Look at the following reaction:
2Al(s) + 3CuCl₂(aq) → 2AlCl3(aq) + 3Cu(s)
This is an example of what type of reaction?
O Single replacement
O Double replacement
ODecomposition
O Synthesis
Answers
Answer:
Single replacement
Explanation: Al is replacing Cu
Question.04: (3mrks) A Manometer is a device to measure the pressure of an enclosed d gas sample. A common simple manometer consists of a U shaped tube of glass filled with some liquid. Typically, the liquid is mercury because of its high density. Incandescent light bulbs "burn out" because their tungsten filament evaporates, weakening the thin wire until it breaks. Argon gas is added inside the bulbs to reduce the rate of evaporation. (Argon is chosen because, as a nobi gas, it will not react with the components of the bulb, and because it is easy to obtain in significant quantities. It is the third most abundant element in air.) What is the pressure in atmospheres of 3.4 x 10-³ moles of argon gas in a 75mL incandescent light bulb at 20 °C?
Answers
The pressure of atmospheres of the argon gas in the given incandescent light bulb is 1. 1 .
How to find the pressure of atmospheres ?The pressure of atmospheres can be found by the formula :
= ( Number of moles x Universal gas constant x Temperature in Kelvin ) / Volume of gas
Number of moles = 3.4 x 10 ⁻³
Universal gas constant = 0. 082
Temperature in Kelvin = 20 + 273. 15 = 293. 15 K
Volume of gas : 75 x 10 ⁻³
The pressure of atmospheres of the argon gas is:
= ( 3.4 x 10 ⁻³ x 0. 082 x 293. 15 ) / 75 x 10 ⁻³
= 1. 1 atm
Find out more on pressures of atmospheres at https://brainly.com/question/19587559
#SPJ1
O CHEMICAL BONDING = Drawing Lewis structures for simple organic compounds Draw the Lewis structure for iodoethane (C2H,1). Be certain you include any lone pairs. Ć c с IT ?
Answers
The Lewis structure for iodoethane (C2H,1) is as follows.
I - C - C - H
| |
H H
| |
H H
What is a Lewis structure?A Lewis structure, also known as a Lewis dot diagram, is a way to represent the bonding and non-bonding electrons in a molecule. It is named after Gilbert N. Lewis, who introduced it in 1916. A Lewis structure shows the chemical symbol of each atom in the molecule and the electrons that are involved in the chemical bonds. It is used to predict the molecular geometry, reactivity, and other chemical properties of a molecule.
In this structure, the central atom is the carbon atom, and the outer atoms are the hydrogen and iodine atoms. The carbon atoms each have four bonds, which are satisfied by the three hydrogen atoms and the iodine atom. The iodine atom has one lone pair, which is not shown in the structure.
To learn more about Lewis's structure visit:
brainly.com/question/20300458
#SPJ4
the table gives the composition of three particles
(a) what is the evidence in the table for each of the following?
(i)Particle A is an atom
(ii) A,B and C are all particles of the same element.
(iii) Particles A and C are isotopes of the same element.
(b) (i) What is the electronic structure of particle A?
(ii) Is element A , a metal or non-metal? Give a reason for your choice

Answers
The evidence in the table for each of the following atoms of an element A, B, and C is that A,B, and C are all particles of the same element; option ii.
What are elements?Elements are substances that are composed entirely of the same atoms and which cannot be split into simpler units by an ordinary chemical process.
Atoms of the same element have the same number of protons or the same atomic number.
However, atoms of the same element may have different numbers of neutrons, and these atoms of the element are called isotopes.
Learn more about elements at: https://brainly.com/question/6258301
#SPJ1
Q1. What is the enthalpy change during the process in which 100.g of water at 50.0°C is cooled
to ice at -30 °C? Show your work to receive full credit. Specific heat of fusion of water = 6.01
kj/mol. Specific heat of ice = 2.03 J/g c.
Answers
The following equation is used to calculate the change in enthalpy that occurs when 100 g of water at 50.0 °C is cooled to ice at -30 °C: First, using the equation q = mcT, it is determined how much heat energy is needed to cool the water from 50.0°C to 0°C. q = (100 g)(4.18 J/g°C)(50.0°C-0°C) = 20900 J in this scenario.
Then, using the equation q = mL, where q is the heat energy, m is the mass of the water, and L is the specific heat of fusion of water, it is determined how much heat energy is needed to turn the water into ice at 0°C. That is. q = (100 g)(6.01 kJ/mol) = 601 kJ in this instance.
The equation q = mcT, where q is the heat energy, m is the mass of the ice, c is the specific heat of ice, and T is the change in temperature, is used to determine the amount of heat energy needed to cool the ice from 0°C to -30°C. q = (100 g)(2.03 J/g°C)(0°C-30°C) = -6090 J in this scenario.
Therefore, the sum of the three heat energy calculations, i.e. 20900 J + 601 kJ - 6090 J = 54110 J, is used to compute the enthalpy change throughout the process in which 100 g of water at 50.0 °C is cooled to ice at -30 °C.
Learn more about enthalpy at:
https://brainly.com/question/16720480
#SPJ1
The different possible ways for arranging the particles of a system are called _____. The greater the number of these states, the _____ the entropy of the system
Answers
The different possible ways of arranging the particles of a system are called states. The greater the number of these states, the higher the entropy of the system.
By ascribing definite values to a satisfactory amount of variables, one can define the state of a system. In simple terms, it is the description of a system condition in terms of properties that are measurable or observable, for example, pressure, temperature, etc.
Entropy is a measure of the disorder or randomness in a system, and an increase in the number of states corresponds to an increase in entropy. The S.I. unit for entropy is joules per kelvin. Entropy is a measurable physical property. In a thermodynamic system, it is an extensive property.
Example: There is an increase in entropy when a block of ice melts.
To learn more about entropy, visit: https://brainly.com/question/15025401
#SPJ11
A sample of matter is made up of three different atoms that are chemically combined. What
type of matter is it? How do you know?
Answers
Answer:
liquid
Explanation:
a molecule consists of different atoms chemically combined. A molecule is also a liquid. Molecules make up ice cubes, which are also small water particles which arr yet again liquid.
What is the main side effect of lithium?
Answers
Lithium is a medication commonly used to treat bipolar disorder, which is a mental health condition characterized by episodes of mania and depression. While lithium is effective in managing the symptoms of bipolar disorder, it can also cause a range of side effects. The main side effect of lithium is its potential to cause lithium toxicity, which can have serious consequences if not properly managed.
Lithium toxicity occurs when there is too much lithium in the body, which can happen if the dosage is too high or if the patient's kidneys are not functioning properly. Symptoms of lithium toxicity can range from mild to severe and may include:
Nausea and vomitingDiarrheaTremorsDrowsinessConfusionSeizuresComaLithium levels in the blood should be closely monitored when taking this medication, as it is important to maintain a therapeutic level of lithium in the blood to effectively treat bipolar disorder, but also to avoid toxicity. If toxicity occurs, the medication should be discontinued immediately and the person should be treated in a hospital setting. This may include the use of a medication called a diuretic, which helps the kidneys excrete excess lithium from the body.
It is important to note that not all people experience these side effects. Also, most people who take lithium do not experience any major side effects and are able to manage their condition effectively with this medication.
Additionally, other side effects can occur, such as weight gain, acne, dandruff, fine hand tremor, dry mouth, polyuria, polydipsia, and hypothyroidism. It's important to talk with your healthcare provider about the potential side effects of lithium and to closely monitor your symptoms while taking this medication.
Here you can learn more about bipolar disorder
https://brainly.com/question/5788499#
#SJP11
What is the∆S° of 0₂
Answers
Answer:0
Explanation: zero because it is the most stable form of oxygen in its standard state
10. What is the density of a rock with a volume of 5 cubic centimeters and a mass of 3 grams?
Answers
Answer:
0.6 g/cm³Explanation:
The density of a substance can be found by using the formula
\(density = \frac{mass}{volume} \\ \)
From the question we have
\(density = \frac{3}{5} \\ \)
We have the final answer as
0.6 g/cm³Hope this helps you
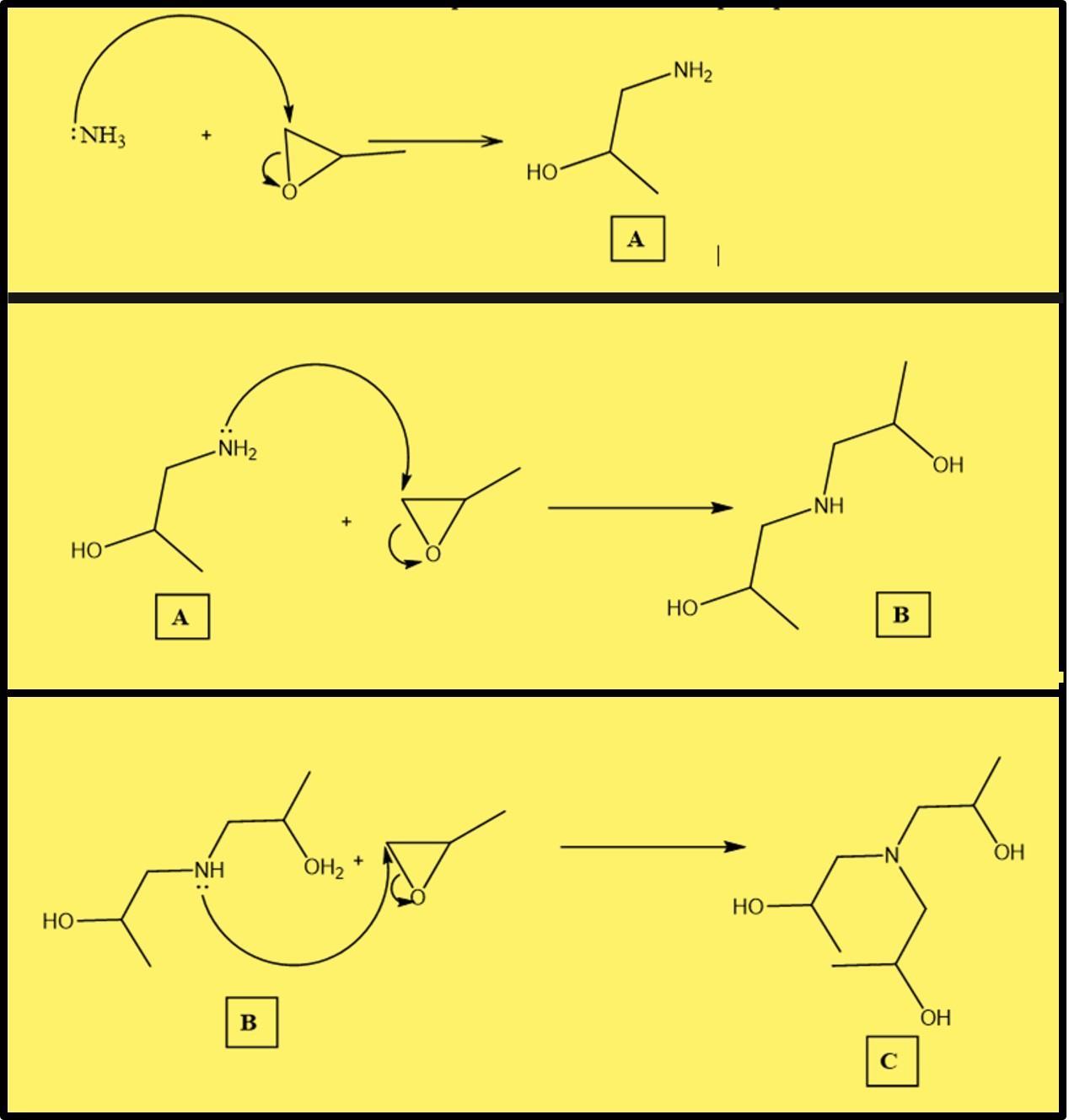
Amines suitable for preparing buffers are often synthesized by treating ammonia with epoxides. Consider the following series of reactions, and draw the structure of compound B.
Answers
It is correct to state that Amines are suitable for preparing buffers are often synthesized by treating ammonia with epoxides. The relative structures of compound B are attached accordingly.
What is an Amine?Amine is a family of basic chemical compounds formed by replacing hydrogen with one or more monovalent hydrocarbon radicals.
Apart from medications and pharmaceuticals, amines are employed in the production of azo-dyes and nylon.
They are commonly employed in the development of crop protection, pharmaceutical, and water purification chemicals. They are also used in personal care products.
Ethanol amines are the most widely utilized form of amine on the global market.
It should be noted that ammonia attacks epoxides at the least substituted carbon to produce (A.)
The generated product is then made to react with another epoxide, resulting in the synthesis of successive products.
Learn more about Amines at;
https://brainly.com/question/17278249
#SPJ1
Consider the
solubility curve at
right. Which SOLID
has the lowest
solubility at 10°C?
A. Substance C
C. Substance D
100
90
Solute per 100 g of H₂O (g)
70
60
50
40
O
0
0 10 20 30 40 50 60 70 80 90 100
Temperature (°C)
B. Substance B
D. Substance A

Answers
The solid that has the lowest solubility at 10°C is substance D
What is the solubility curve?A solubility curve is a graphic representation of a solute's solubility in a particular solvent at different pressures and temperatures. A material's solubility, which is often expressed in grams per 100 milliliters (g/100 mL) of solvent, is the maximum quantity of the substance that can dissolve in a given amount of solvent at a particular temperature and pressure.
This is because, the solubility of the substance D as we can see from the curve is closest to zero around 10°C .
Learn more about solubility curve:https://brainly.com/question/14366471
#SPJ1
Answer:
Substance D
Explanation:
its right on acellus
How do the ramp heights of the different objects compare? How does the ramp height relate to the strength of the frictional force between the book and the object?
Answers
The height of a ramp does not directly determine the strength of the frictional force between a book and an object.
How do they compare?The strength of the frictional force between a book and an object is not directly influenced by the height of a ramp. The nature of the surfaces in contact, the force forcing the surfaces together (normal force), and the coefficient of friction are some of the variables that affect the frictional force between two surfaces.
The coefficient of friction between the book and the object plays a major role in determining the strength of the frictional force.
Learn more about frictional force:https://brainly.com/question/30280206
#SPJ1
Which is a postulate of the kinetic-molecular theory?
O Gas particles have a small volume relative to the spaces between them.
Gas particles have a large volume relative to the spaces between them.
Gas particles are very small in size and always move slowly.
O Gas particles are very large in size and always move slowly.
Answers
Answer:
Gas particles have a small volume relative to the spaces between them.
Explanation:
i just took the quiz.
Gas particles have a small volume relative to the spaces between them is the postulate of the kinetic molecular theory.
What is kinetic molecular theory?The kinetic molecular theory describes about the behaviour of ideal gases at the particle level.
The five main postulates of the kinetic molecular theory are as follows:
1) The particles in a gas are in constant and random motion
2) The combined volume of the particles is negligible.
3) The particles exert no forces on one another.
4) The collisions between the particles are completely elastic.
5) The average kinetic energy of the particles are proportional to the temperature in kelvin.
Hence, we can conclude that option 1 is the answer.
To learn more about kinetic molecular theory here
https://brainly.com/question/10725862
#SPJ2
14.600 oz = ____g
(HELP ME PLEASE)
Answers
Answer:
The answer is 414.64 g the other answer is wrong
Explanation:
I took this assignment and got this question right
Pt 2. Chem Reactions 50 PTS
Hey, I need help with Chemistry. Please also provide an explanation as well!!



Answers
The red colour is the limiting reactant.
Red-blue colour ball and two white balls attached together are reactants.
Red-blue colour ball and two white and one red colour ball attached to each other are products.
What is a limiting reagent?The reactant that is entirely used up in a reaction is called a limiting reagent.
A reactant is a substance that is present at the start of a chemical reaction. The substance(s) to the right of the arrow are called products.
A product is a substance that is present at the end of a chemical reaction.
Hence,
The red colour is the limiting reactant.
Red-blue colour ball and two white balls attached together are reactants.
Red-blue colour ball and two white and one red colour ball attached to each other are products.
Learn more about limiting reagents here:
brainly.com/question/26905271
#SPJ1
examples of metal non-metal and semi metal
Answers
Answer:
Semi : Boron [B] Non-Metal : Carbon [C] Metal : Manganese [Mn]
Explanation:
Periodic table I'm guessing!
How many electrons are there in .5 grams of Aluminum
Answers
The number of moles in 0.5 grams of Aluminum = 0.5 / 26.9815= 0.018521Let us now use Avogadro's number to find the number of atoms in 0.5 grams of Aluminum. 0.5 grams of Aluminum contain 3.01 × 10^22 electrons.
let us begin by converting the mass of Aluminum given to number of moles. We can use the following equation for this:moles = mass / molar mass of AluminumThe molar mass of Aluminum is 26.9815 g/molThere are 6.022 × 10²³ atoms in one mole of any element.
The number of atoms of Aluminum = number of moles × Avogadro's number= 0.018521 × 6.022 × 10²³= 1.115 × 10²²Now, we know that one atom of Aluminum contains 13 electrons. So, the total number of electrons in 1.115 × 10²² atoms of Aluminum= 1.115 × 10²² × 13= 1.4475 × 10²³So, 0.5 grams of Aluminum contains 1.4475 × 10²³ electrons.
To know more about Aluminum visit:
https://brainly.com/question/28989771
#SPJ11
Which animal is found in the desert?
monkeys
parrots
buffalo
meerkats
Answers
Answer:
meerkats
Explanation:
Uranium contains two isotopes, U-235 with an atomic mas of 235 g/mol, and u-238 with an atomic mass of 238g/mol. U-235 is needed
as a fuel in nuclear reactors. Until recently, the method used to separate U-235 from U-238 was by gas diffusion. Use U-235 as R1, and
U-238 as R2 and determine the rate of diffusion and which gas will diffuse faster.
⚪︎U-235 diffused 10.01 times faster than U-238
⚪︎U-235 diffused 1.01 times slower than U-238
⚪︎U-235 effused 2.01 times slower than U-238
⚪︎U-235 effused 1.01 times faster than U-238
⚪︎U-235 diffused 1.01 times faster than U-238

Answers
Answer:
the answer is in the image
Explanation:

igcse 2023 past papers edexcel, does anyone have the papers..? pls upload if do, i want chem, human bio, maths and physics,pls if u hv a big help
Answers
Unfortunately, the Edexcel IGCSE 2023 past papers are not yet available. We suggest that you check the Edexcel website periodically for updates.
What is available ?
There are many types of items available for purchase. Consumers can find a wide variety of goods, from clothing and electronics to food and furniture. Online retailers offer a convenient way to shop, with many items available for delivery or pick up. Consumers may also find items from local stores or through online auctions. Additionally, products can be purchased through subscription services, such as meal kits or beauty boxes. Finally, services such as home cleaning, pet sitting, and tutoring can also be purchased.
To learn more about available
https://brainly.com/question/11231920
#SPJ1
For a certain polyatomic ideal gas the value of its ideal gas constant is 0.123 kJ/(kg.K). Determine a) its molecular weight (W);
Answers
The molecular weight (W) of the polyatomic ideal gas is equal to the temperature (T) divided by the volume (V) calculated as 0.123 kJ/(K).
The molecular weight (W) of the polyatomic ideal gas can be determined using the ideal gas equation:
PV = mRT
where:
P = pressure of the gas (in this case, it is not given)
V = volume of the gas (in this case, it is not given)
m = mass of the gas (in kilograms)
R = ideal gas constant (0.123 kJ/(kg.K))
T = temperature of the gas (in Kelvin)
To calculate the molecular weight (W), we need to find the value of m. Since the pressure and volume are not provided, we can rearrange the ideal gas equation as follows:
m = PV / (RT)
Now, let's assume a hypothetical situation where we have 1 kg of the polyatomic ideal gas. In this case, the mass (m) would be equal to 1 kg.
Substituting the values into the equation:
m = (1 kg) * V / (0.123 kJ/(kg.K) * T)
Here, we can see that the units of kilograms (kg) cancel out, leaving us with:
1 = V / (0.123 kJ/(K))
To isolate V, we multiply both sides of the equation by 0.123 kJ/(K):
0.123 kJ/(K) = V
Now, we have the volume (V) in cubic meters. The molecular weight (W) can be calculated using Avogadro's law, which states that equal volumes of gases, at the same temperature and pressure, contain an equal number of molecules.
To calculate the molecular weight (W), we need to determine the number of moles (n) of the gas. The number of moles can be found using the equation:
n = PV / (RT)
However, since the pressure and volume are not provided, we cannot calculate the number of moles directly. Instead, we can make use of the molar mass (M) of the gas, which is the mass of 1 mole of the gas.
The molar mass (M) is related to the molecular weight (W) as follows:
M = W / 1000
Since we assumed a mass of 1 kg earlier, the molar mass (M) can be calculated as:
M = (1 kg) / n
Substituting the value of n from the equation above:
M = (1 kg) / (PV / (RT))
M = RT / PV
Now, substituting the value of R (0.123 kJ/(kg.K)) and rearranging the equation:
M = (0.123 kJ/(kg.K)) * T / (0.123 kJ/(K) * V)
The units of kJ cancel out, leaving us with:
M = T / V
Using the value of V we calculated earlier (0.123 kJ/(K)), we can determine the molecular weight (W) of the polyatomic ideal gas.
To know more about weight visit:
https://brainly.com/question/27988184
#SPJ11
What does Aluminum and Magnesium oxide create
Answers
Answer: MgAl2O4 or Spinel (gemstone)
Explanation:
7. What is the weather of a certain area over a long period of time?
(10 Points)
climate zone
element
climate
O moisture

Answers
Answer:
Climate
Explanation:
1. How many gram of calcium chloride are formed when 15g of calcim metal react with hydrochloric acid.
2.balance the following equation by the lcm and inspection method.
a. pcl +H2o= =H3po4 +Hcl
b. c +o2 =co2
3. How many moles of H2o are required to produce 4.5 moles of HNO3 According to the following reaction 3NO2 + H2o = 2HNO3 + No
please freind step and correct answer thank you !
Answers
Mole measure the number of elementary entities of a given substance that are present in a given sample. Therefore, 83.23g of calcium chloride are formed when 15g of calcium metal react with hydrochloric acid.
What is mole?The SI unit of amount of substance in chemistry is mole. The mole is used to measure the quantity or amount of substance. We know one mole of any element contains 6.022×10²³ atoms which is also called Avogadro number.
mole of calcium=15g/ 20
=0.75moles
Ca +2HCl → CaCl\(_2\) + H\(_2\)
thee mole ratio between calcium and calcium chloride is 1:1
mole of calcium chloride=0.75moles
mass of calcium chloride= 0.75moles ×110.983 g/mol.
=83.23g
Therefore, 83.23g of calcium chloride are formed when 15g of calcium metal react with hydrochloric acid.
To know more about mole, here:
https://brainly.com/question/15209553
#SPJ2